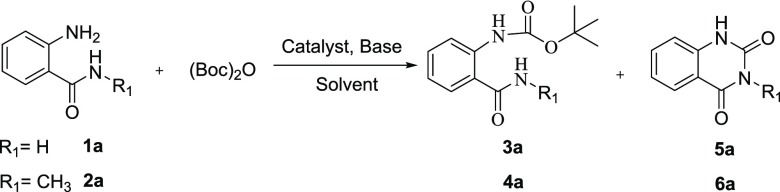
Table 1. Optimization of Reaction Conditionsa.
| yield (%) |
||||||
|---|---|---|---|---|---|---|
| entry | R1 | catalyst | base | solvent | 3a/4a | 5a/6a |
| 1b | H | DMAP | Et3N | CH2Cl2 | 9 | 33 |
| 2b | H | Et3N | CH2Cl2 | 9 | 13 | |
| 3b | H | DMAP | CH2Cl2 | 79 | ||
| 4b | H | CH2Cl2 | 10 | 47 | ||
| 5b | H | TBD | CH2Cl2 | NRe | ||
| 6b | H | DBU | CH2Cl2 | NRe | ||
| 7b | H | DABCO | CH2Cl2 | 36 | ||
| 8b | H | DMAP | THF | 58 | ||
| 9b | H | DMAP | DMF | 61 | ||
| 10b | H | DMAP | CH3CN | 94 | ||
| 11b | CH3 | DMAP | CH3CN | 46 | 46 | |
| 12c | CH3 | DMAP | CH3CN | 21 | 59 | |
| 13d | CH3 | DMAP | CH3CN | 92 | ||
All reactions were conducted using 1a/2a (1 mmol, 1.0 equiv), (Boc)2O (1.5 mmol, 1.5 equiv), catalyst (0.1 mmol, 0.1 equiv), solvent (3 mL), isolated yield.
The reaction was run at room temperature for 12 h.
The reaction was run at reflux for 12 h.
The reaction was run under the microwave (MW) condition for 30 min.
No reaction.