Abstract
Actigraphy, a method for inferring sleep/wake patterns based on movement data gathered using actigraphs, is increasingly used in population-based epidemiologic studies because of its ability to monitor activity in natural settings. Using special software, actigraphic data are analyzed to estimate a range of sleep parameters. To date, despite extensive application of actigraphs in sleep research, published literature specifically detailing the methodology for derivation of sleep parameters is lacking; such information is critical for the appropriate analysis and interpretation of actigraphy data. Reporting of sleep parameters has also been inconsistent across studies, likely reflecting the lack of consensus regarding the definition of sleep onset and offset. In addition, actigraphy data are generally underutilized, with only a fraction of the sleep parameters generated through actigraphy routinely used in current sleep research. The objectives of this paper are to review existing algorithms used to estimate sleep/wake cycles from movement data, demonstrate the rules/methods used for estimating sleep parameters, provide clear technical definitions of the parameters, and suggest potential new measures that reflect intraindividual variability. Utilizing original data collected using Motionlogger Sleep Watch (Ambulatory Monitoring Inc., Ardsley, NY), we detail the methodology and derivation of 29 nocturnal sleep parameters, including those both widely and rarely utilized in research. By improving understanding of the actigraphy process, the information provided in this paper may help: ensure appropriate use and interpretation of sleep parameters in future studies; enable the recalibration of sleep parameters to address specific goals; inform the development of new measures; and increase the breadth of sleep parameters used.
Keywords: actigraphy, Motionlogger Sleep Watch, sleep parameters, sleep/wake algorithms
Introduction
Actigraphy, a method for inferring sleep/wake cycles based on magnitude of wrist movement collected using digital devices called actigraphs, has been used for over two decades in studies of sleep and circadian rhythms (Cole et al., 1992; Ancoli Israel et al., 2003; Van de Water et al., 2011). Although polysomnography (PSG) is considered the gold standard for assessment of sleep (Van de Water et al., 2011), the procedure is costly, invasive, and disruptive to participants’ normal sleep routines (Blackwell et al., 2008; Van de Water et al., 2011), making it impractical for application in population-based epidemiologic studies. In contrast, actigraphy has properties that render it useful for collecting objective sleep data in large-scale investigations (Ancoli Israel et al., 2003; Littner et al., 2003; Blackwell et al., 2008; Martin and Hakim, 2011). Notably, actigraphy is more convenient, less invasive, and lower cost than PSG, and the device can be worn continuously, 24 h a day, for extended period of time (days, weeks, or even longer). In addition, collection of actigraphic data over multiple nights in the participant’s natural environment can provide more reliable estimates of sleep compared to PSG, which is typically performed for only one or two nights in a sleep laboratory (Berger et al., 2007; Blackwell et al., 2008; Rupp and Balkin, 2011). Importantly, actigraphy has been validated (against PSG) in multiple populations (Cole et al., 1992; Sadeh et al., 1994; Jean-Louis et al., 1997a,b; 2001; de Souza et al., 2003; Lichstein et al., 2006; Blackwell et al., 2008; Tonetti et al., 2008; Martin and Hakim, 2011; Rupp and Balkin, 2011; Van de Water et al., 2011; Meltzer et al., 2012a,b). In a comprehensive review of the role of actigraphy in studies of sleep and circadian rhythms, Ancoli Israel et al. (2003) reported that, in adult populations, the estimated agreement between PSG and actigraphy ranged from 91 to 93%.
Actigraphs are small watch-shaped devices that are generally placed on the non-dominant wrist and contain motion detectors (accelerometers) to monitor and record movements (Ancoli Israel et al., 2003). Although there are variety of commercially available actigraphs (Sadeh, 2011), the Motionlogger Sleep Watch manufactured by Ambulatory Monitoring Inc. (AMI, Ardsley, NY) is among the commonly used devices in epidemiologic and laboratory studies (Meltzer et al., 2012a,b; Bellone et al., 2016). Generally, actigraphs are worn for several days, during which time the device records movement data multiple times per second and stores the information for each minute of a day, resulting in 1440 observations (wrist movement data points) per 24-h period. The data are then downloaded to a computer where specialized software (Action-W, AMI, Ardsley, New York) is used to automatically assess sleep/wake cycles based on an algorithm and then estimate sleep parameters separately for each day or a 24-h period (Martin and Hakim, 2011). The sleep estimates are then averaged across days to derive a more stable measure for the participant. Although the software generates about two dozen sleep parameters, very few are typically utilized in most sleep research (Ancoli Israel et al., 2003), namely, total sleep time, sleep efficiency (SE), sleep latency, wake after sleep onset (WASO), and number of awakenings (de Souza et al., 2003; Berger et al., 2005; Berger et al., 2007; Blackwell et al., 2008; Natale et al., 2009; Tranah et al., 2010; Natale et al., 2014).
While there is abundant evidence on application of actigraphy in both research and clinical settings (Ancoli Israel et al., 2003; Natale et al., 2009; Meltzer et al., 2012a,b; Fawkes et al., 2015), specific literature demonstrating the methodology involved in deriving the various sleep parameters from the digital counts stored by actigraphs, is limited. In addition, there is lack of consensus in definition of sleep onset and offset, which then leads to inconsistent reporting of sleep parameters across studies (Berger et al., 2005; Smith et al., 2018). Understanding the methods used for deriving the sleep parameters from wrist movement data is essential for making informed decisions regarding the interpretation of the parameters and their appropriate use. Due to the absence of literature detailing how sleep parameters are derived, the process often remains essentially a black box, especially to those not directly involved in this line of research. Enhancing the understanding of the methodology involved in the generation of sleep measures from actigraphy may (i) improve the interpretation of the derived parameters and hence, their appropriate application in future studies, (ii) enable researchers to revise the definition of some sleep parameters to better address a specific objective [e.g. what constitutes a long wake or sleep episode (SEP), what constitutes latency to persistent sleep (LPS), etc.], (iii) broaden the use of sleep parameters that have often been ignored [e.g. sleep fragmentation index (SFX), brief wake ratio (BWR), short burst inactivity index (SBIX), intraindividual variability in sleep parameters], and (iv) lead to development of new sleep measures. Therefore, the objectives of this study were to (i) review and illustrate existing algorithms used to estimate sleep/wake cycles from movement data, (ii) demonstrate the rules/methods used for estimating sleep parameters from wrist movement data, (iii) provide clear technical definitions of the derived sleep parameters, and (iv) suggest potential new measures that reflect intraindividual variability. We attempt to clarify how the input (movement data recorded by actigraph) is translated into output (sleep measures estimated by actigraphic software) such that readers/users have a more comprehensive understanding of the process involved. Although this paper is primarily intended for users of actigraphy who have little to no experience in the methodology involved, we believe it will serve as a useful resource for intermediate to advanced users as well. Although the methodology discussed is based on the Motionlogger Sleep Watch (AMI, NY) and the associated analytic software (Action-W), the information presented will be useful for understanding sleep measures derived using other commonly used actigraphs such as the Mini-Mitter actigraphs (now owned by Phillips-Respironics, Bend, Oregon) and Cambridge Actiwatch actigraphs (Cambridge, UK).
In this paper, we first detail the methodology in actigraphy including (i) actigraphic data modalities or types of raw data (often referred to as ‘activity counts’) generated by the Motionlogger actigraph, (ii) data preprocessing, (iii) sleep scoring algorithms, (iv) identification of time in bed (TIB) and sleep period (SLP) with emphasis on technical definitions that mark the end points of these two intervals, and (v) technical definitions of sleep parameters derived from actigraphic data along with our suggestion of potential new measures. We then present a demonstration of actigraphy methodology using supplemental actigraphic data.
Methodology in actigraphy
Actigraphic data modalities
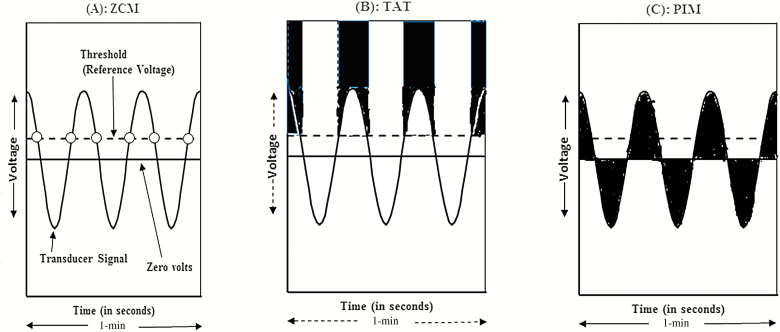
The actigraph, which contains an accelerometer for detection of movement, produces a continuous electrical signal (voltage) when a participant wearing the device is in motion. After motion is transduced into an analog electrical form, samples are collected from this continuous signal at the rate of 10 hertz (10 Hz)—that is, 10 samples per second. The voltage signals sampled are processed in three different ways: Zero Crossing Mode (ZCM), Time Above Threshold (TAT), and Proportional Integration Mode (PIM), to provide information on movement frequency, duration, and intensity, respectively. The extracted information is then digitized (stored) as data points at 1-min recording intervals (Jean-Louis et al., 2001; Ancoli Israel et al., 2003; Hersen, 2006; Blackwell et al., 2008; Tranah et al., 2010; Rupp and Balkin, 2011); the user can specify the recording interval anywhere from 1 s to 10 min, but a length of 1 min is the standard for most applications. A visual heuristic of the three modes of operation is illustrated in Fig. 1 and an excellent discussion of these data types can be found elsewhere (Hersen, 2006). The ZCM (Fig. 1A) yields a count of the number of times per minute the transducer signal (i.e. voltage) crosses a reference voltage (a preset threshold) which usually is a value set close to zero (Ancoli Israel et al., 2003; Hersen, 2006). In Fig. 1A, the value of ‘activity count’ that would be stored in the memory of the actigraph for that minute would be 6. In TAT Mode (Fig. 1B), the amount of time, in tenths of a second, spent above the sensitivity threshold is accumulated during a minute. TAT is a measure of time spent in motion or duty cycle (Hersen, 2006). The Proportional Integrating Measure Mode is a high-resolution measurement of area under the transducer signal (Fig. 1C). It involves sampling the output signal at a high rate and then calculating the area under the curve for each minute (Ancoli Israel et al., 2003). The PIM is a measure of activity level or vigor of motion (Ancoli Israel et al., 2003; Hersen, 2006). The type of movement data (mode) used for estimation of sleep parameters depends on a number of factors including age, gender, underlying disease of the study population, and the research question (Ancoli Israel et al., 2003; Hersen, 2006; Blackwell et al., 2008; Tranah et al., 2010). Generally, ZCM is chosen as the mode of operation, especially in sleep research, because of its ability to estimate sleep with a high degree of accuracy.
Figure 1.
Diagrammatic representation of the three modes in which wrist movement data is digitized and stored by the Motionlogger Sleep Watch actigraph (i.e. the three types of activity counts or movement data generated by the device). Panel A, the ZCM represents the number of times per epoch the voltage signal (represented by the solid curved line) crosses a threshold (dashed horizontal line). Panel B, the TAT mode is the amount of time (in tenths of a second) the voltage signal is above a threshold. Panel C, the Proportional Integrating Measure (PIM) mode represents the area under the curve for each epoch. The figure is adapted from Jean-Louis et al. (2001).
Data preprocessing
Although not the subject of this study, data preprocessing (data editing) is an important step in the analysis of actigraphy data (Tranah et al., 2010; Natale et al., 2014). Data editing includes (i) trimming of non-data from the beginning and end of a file, which keeps or marks the section of data one wants to use for analysis, (ii) identifying and marking ‘off-wrist’ segments (i.e. non-wear periods) to exclude these intervals from analyses, and (iii) marking of TIB. Some actigraphic devices have features that enable one to identify ‘off-wrist’ segments (e.g. a life channel that records positive activity count for each minute when the device is worn and zero activity score when the device is off wrist). Most actigraphic devices have an ‘event marker’ that participants push to mark time in and out of bed (Martin and Hakim, 2011) and which allow correct identification of TIB. Light intensity measured by the actigraph and the magnitude of the activity recorded could also aide in accurate identification of TIB.
Sleep diaries, when used concurrently with an actigraph, are also a useful resource for augmenting actigraphy data. For example, sleep scores can be manually edited after they are generated by the software when the analyst has additional information on the subject’s sleep behavior from the sleep diary (User’s Guide to Action4, Version 1.1, 2010; Action-W User’s Guide, 2011). As a result, sleep diary data could potentially improve the estimation of sleep onset latency (SOL) by differentiating awake but motionless from true sleep (Martin and Hakim, 2011). In addition, understanding of the characteristics of the population being studied (e.g. occupation type, work schedule, etc.) can also inform the editing process. For example, shift workers may be bimodal sleepers with two separate bedtimes in a 24-h period. Overall, data editing is a time consuming but crucial step that must be completed prior to deriving actigraphy-based sleep parameters. Sleep diary data are particularly important in determining ‘lights off’ (which signals start of the SLP), information critical for accurate assessment of sleep latency and SE (Smith et al., 2018). A review by Sadeh (2011) presents several examples of studies that indicate strong positive correlations between actigraphic and sleep diary estimates of sleep schedule measures (sleep onset time and sleep offset time). While actigraphy typically identifies numerous isolated brief awakenings which may be common even in normal sleep, awakenings identified via sleep diary likely reflect a distinct construct related to consolidated frank awakenings (Smith et al., 2018); hence, data from sleep logs can play an important role in validating awakenings identified by the sleep algorithms. Overall, a sleep diary record provides additional useful information about sleep patterns and has been shown to be an important adjunct to actigraphy for editing data (especially identification of TIB) and for removing artifacts. Furthermore, sleep diary data would enable investigators to compare participant perceptions of sleep (self-report) with objective sleep estimates from actigraphy. It is recommended that sleep logs be collected concurrently with actigraphy. However, in actigraphy studies that do not involve collection of sleep diary data, investigators should consider providing detailed instruction to participants, including that regarding the event-marker feature in the actigraph and the times participants need to use the event marker to identify important events (when first lying in bed, when lights are off, and when getting up); the importance of properly marking these events/points to the study objective should be emphasized to the study participants.
Sleep scoring
Sleep scoring algorithms
How do we estimate sleep/wake cycles from activity data? Sleep estimation algorithms (also known as sleep scoring functions) are mathematical expressions that utilize the activity data (wrist movement values) as input and determine whether the subject wearing the device was awake (coded as 0) or asleep (coded as 1) during each minute where activity data were collected. The algorithm basically transforms the wrist movement data (often called ‘activity counts’) into a series of 0’s and 1’s (often referred to as ‘sleep scores’ or ‘sleep/wake scores’). There are various validated algorithms for estimating sleep/wakefulness based on wrist movement data collected using the actigraphs from AMI (Cole et al., 1992; Sadeh et al., 1994; Jean-Louis et al., 2001). Generally, the algorithm employed for scoring sleep depends on (i) the device used for monitoring movement; (ii) the mode of operation (data type); (iii) epoch length; and (iv) age of the study population. The Cole–Kripke algorithm (Cole et al., 1992) and the University of California, San Diego (UCSD) scoring algorithm (Jean-Louis et al., 2001) are the most widely used sleep scoring functions. In adult populations, the Cole–Kripke and UCSD algorithms can be used to assess sleep/wake cycles for data collected in the ZCM mode, while the UCSD scoring algorithm is the only available algorithm for data collected in the PIM and TAT modes. To score sleep during a specific 1 min, these sleep scoring functions use the activity scores for the 4 min preceding the minute of activity under consideration, the actual minute being scored, and the 2 succeeding minutes (Cole et al., 1992; Jean-Louis et al., 2001). The algorithm then weighs the activity data from each of these seven time points using predetermined coefficients (also called weighting coefficients) and computes the sum of the weighted values. The entire sum is multiplied by the constant P (scaling factor). The current minute is scored as sleep if the resulting value is lower than 1, and as awake if the value is ≥1. This process can be stated using the following mathematical expression: , where A−4 to A−1 are the activity scores for the 4 min preceding the minute of activity under consideration, A0 is the activity score for current minute (the minute being scored), A+1 to A+2 are the activity scores for the two succeeding minutes, wa−4 to wa+2 are the weights (coefficients) applied to each activity minute, and P is the scaling factor. The sleep score for the current minute is assigned a value of 1 (if S < 1) or zero (if S ≥ 1). In short, these sleep scoring algorithms calculate a moving average, which takes into account the activity levels immediately prior to and after the current minute to determine if each time point should be coded as sleep or awake. The equation for scoring sleep, using the Cole–Kripke or UCSD algorithms, based on activity data collected via the Zero Crossing Mode (ZCM) is as follows: S = 0.0033 × (1.06. × A−4 + 0.54 × A−3 + 0.58 × A−2 + 0.76 × A−1 + 2.30 × A−0 + 0.74 × A+1 + 0.67 × A+2. If S < 1 then the subject is coded asleep (score of 1) at the particular minute, awake (score of 0) otherwise. In a validation study of healthy subjects (de Souza et al., 2003), the Cole–Kripke algorithm was shown to correctly distinguish sleep from wakefulness with a high degree of accuracy (91%) and high sensitivity (99%).
Alternatively, the Sadeh algorithm (Sadeh et al., 1994) scores sleep using a different mathematical function as follows: PS = 7.601 − (0.065. × MA5) − (1.08 × NAT) − (0.056 × SDA6) − (0.073 × In ( A0 + 1)), where PS is probability of sleep, MA5 is the mean activity score during the scored minute, the five preceding minutes, and the five following minutes (11-min window), NAT is the number of minutes with activity score ≥50 and <100 in the same 11-min window, SDA6 is the standard deviation of the activity score during the scored minute and the 5 min preceding it (6-min window), ln(A0 + 1) is the natural logarithm of the activity score for the minute being scored plus 1. If PS ≥0, then the subject is coded asleep at the particular minute, awake otherwise. The Sadeh algorithm has also been shown to have high accuracy (91%) and high sensitivity (97%) compared to PSG (de Souza et al., 2003).
It is worth noting that the Mini-Mitter actigraph employs a similar approach for sleep scoring (Respironics, 2018). For activity data collected at 1-min interval, the Actiware software (Respironics, Inc.) scores each minute as sleep or wake using a validated algorithm that utilizes the activity score of the minute being scored as well as those immediately surrounding it (the 2-min preceding and following the current minute). The total activity count (T) during the 5-min window is calculated as follows: T = (wa−2 × A−2) + wa−1 × A−1) + wa−0 × A−0) + wa+1 × A+1) + wa+2 × A+2), where A−2 and A−1 are the activity scores for the 2 min preceding the minute of activity under consideration, A0 is the activity score for current minute (the minute being scored), A+1 to A+2 are the activity scores for the two succeeding minutes, wa−2 to wa+2 are the coefficients applied to each activity minute. The total activity count (T) is compared to a threshold value set by the researcher and the minute is scored awake if the total activity count exceeds the threshold; Sleep score for the current minute is assigned a value of 1 (if T > threshold) or zero (if T ≤ threshold). The researcher can choose one of three commonly used wake threshold values: low (20), medium (40), or high (80).
Sleep rescoring
The original sleep scores generated by the algorithm are rescored in certain scenarios. The algorithm incorrectly scores actual wake as sleep more often than it incorrectly scores sleep as wake (Cole et al., 1992), in part due to the fact that subjects falling asleep stop moving for few minutes before onset of actual sleep. Therefore, to correct for this misclassification, colleagues (Webster et al., 1982) developed the following five sleep rescoring rules that can be applied to the original sleep scores generated by the algorithm: (i) rescore the first minute of a string of sleep scores to a wake score if preceded by 4 or more minutes of wake. Meaning, rescore 1 min of sleep to wake if the preceding 4 or more minutes were wake (e.g. sleep scores of [0 0 0 0 1] will be changed to [0 0 0 0 0]); (ii) rescore 3 min of sleep as wake if the preceding 10 min were wake (e.g. sleep scores of [0 0 0 0 0 0 0 0 0 0 1 1 1] will be changed to [0 0 0 0 0 0 0 0 0 0 0 0 0]); (iii) rescore 4 min of sleep as wake if the preceding 15 min were wake (e.g. sleep scores of [0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 1 1 1 1] will be changed to [0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0]); (iv) if a period of 6 min or less that is scored as sleep is surrounded (i.e. in both sides) by at least 15 min scored as wake, then rescore to wake (e.g. sleep scores of [0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 1 1 1 1 1 1 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0] will be changed to [0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0]); and (v) if a period of 10 min or less that is scored as sleep is surrounded (i.e. in both sides) by at least 20 min scored as wake, then rescore to wake (e.g. sleep scores of [0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 1 1 1 1 1 1 1 1 1 1 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0] will be changed to [0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0]). Note that users can turn off the sleep rescoring option when analyzing actigrpahic data using the Action-W software.
Identification of TIB and SLP
TIB, also referred to as the major sleep period (Martin and Hakim, 2011), DOWN interval, REST interval, or assumed sleep interval, is the time when subjects are attempting to sleep. TIB is simply the interval from the time the subject went to bed (bedtime) to the time the subject arose (rise time) (Martin and Hakim, 2011). However, the absolute value of TIB is dependent upon user-defined settings for when the subject went to bed (in bed time) and got out of bed (get up time). The end points of TIB (in bed time and get up time) are often ascertained using data from (i) the event marker of the actigraph or (ii) sleep diary, (iii) the ambient light sensor of the actigraph, or (iv) combination of these. It is important to emphasize that studies should clearly state the instructions given to study participants for marking the end points of TIB. In other words, when should TIB begin? Possible choices include: (i) when the participant first lie down in bed but may still read a book or watch TV; (ii) when the participant is in bed and turns the lights off, but may still watch TV; or (iii) when the participant is in bed, turns the lights off, and does nothing except attempting to sleep. Similarly, specifications for ‘get up time’ should be clearly stated and the choices could include (i) the final awakening time but still in bed or (ii) when lights are turned on or (iii) when the subject physically gets out of bed. We recommend that TIB should begin when the subject first lies down in bed and end when the subject physically gets out of bed; this allows derivation of TIB as an independent sleep measure that estimates the amount of time a subject spends in bed regardless of sleep. For proper estimation of sleep latency and efficiency, we also suggest that participants mark when lights are turned off and they do nothing except attempting to sleep.
Sleep period, also referred to as the true sleep period, is defined as the interval (within TIB) from sleep onset to sleep offset (Sadeh, 2011). This interval, also called the sleep onset–offset interval (‘O–O’ interval), represents the period from sleep ONSET (i.e. start of first SEP after getting into bed) to OFFSET (end of the last SEP before getting out of bed). The length of true sleep period is dependent up on the criteria or algorithm used for defining sleep ONSET and sleep OFFSET. The Action-W software (Action-W User’s Guide, 2011) defines Sleep ONSET in three different ways including (i) the time point of the first minute the subject was scored asleep after getting into bed, or (ii) the time point of the first continuous block of at least 10 min of sleep (with no more than 1 min of wake somewhere during the 10 min block), or (iii) the time point of the first continuous block of at least 20 min of sleep (with no more than 1 min of interruption in the 20 min block). Sleep OFFSET on the other hand is defined as the time point of the last minute the subject was scored asleep before getting out of bed.
It is worth noting that sleep parameters can be derived using data from the major sleep period (TIB) or the true sleep period. The Action-W software generates two separate estimates of the sleep parameters, one based on data from TIB and the other using data from the SLP. The interval used for derivation of the values of these sleep parameters should be explicitly stated in all studies.
At this point it is relevant to briefly point out how the major and true sleep periods are defined by the other two commonly used commercial actigraphs, the Mini-Mitter actigraph, (Respironics, 2018) and MotionWatch actigraph (CamNtech, 2018). The Mini-Mitter actigraph and the associated sleep analysis software (Actiware) utilize two approaches for defining sleep interval (i.e. for defining sleep onset and sleep offset/sleep end). The first approach uses the minute-by-minute activity scores to define sleep interval. In this approach, the activity scores at each minute are first classified as immobile (if the activity count recorded at that minute is <4) or mobile (if activity count ≥4). Sleep onset is then defined as the time point of the start of the first 10 consecutive minutes of immobility, after getting to bed, with no more than 1 min of mobility in that interval. Sleep offset is defined as the time point of the last minute of the last 10 consecutive minutes of immobility, before getting out of bed, with no more than 1 min of mobility in that interval. The second approach utilizes the sleep/wake scores (0’s and 1’s) to define sleep interval. Using this approach, sleep onset is the time point of the start of the first 10 consecutive minutes of sleep after getting to bed while sleep offset is the time point of the last minute of the last 10 consecutive minutes of sleep before getting out of bed (Respironics, 2018).
The MotionWatch actigraph and the associated proprietary software (MotionWare) identify the following five time points of interest: ‘in bed time’, ‘lights out time’, ‘fell asleep time’, ‘woke up time’, and ‘got up time’. These time points were defined as follows: ‘In bed time’ was defined as the time point when the subject first lie down in bed, ‘Lights out time’ when the lights are turned off, ‘fell asleep time’ when the subject first fell asleep, ‘woke up time’ when the subject finally awoke before getting out of bed, and ‘got up time’ when the subject finally arose or got out of bed. The interval from ‘lights out time’ to ‘got up time’ is considered the major sleep period (TIB) while the interval from ‘fell asleep time’ to ‘woke up time’ is considered the SLP (sleep region). The software (MotionWare) then derives numerous sleep parameters based on data from sleep region/SLP (the interval from ‘fell asleep time’ to ‘woke up time’), not based on data from TIB.
Overall, both sleep onset and sleep offset are often calculated using specific time-based scoring rules that utilize either the activity counts (i.e. the raw frequency of wrist movement) or the sleep/wake scores (i.e. 0’s and 1’s). The most common definition of sleep onset was the start of the first predetermined number of consecutive minutes of decreased activity count (below a specific threshold) or the start of the first predetermined number of consecutive minutes of sleep; frequently used rules include 1, 3, 5, 10, 15, and 20 consecutive minutes (Meltzer et al., 2012a,b). Similarly, sleep offset was most commonly defined as the last minute of a predetermined number of minutes the subject was scored asleep prior to getting out of bed; frequently used rules include 1, 3, 5, 10, and 15 consecutive minutes. Recommendation as to which definition to use for identifying TIB and SLP should be reached by a consensus from a panel of sleep experts; such decision would enable standardization of actigraphy-based sleep parameters which facilitates comparison of the sleep measures across studies. Finally, we would like to underscore the importance of providing detailing instructions to participants on the use of event markers with emphasis on their importance to study objectives.
Sleep parameters
In this section, we present technical definitions for numerous sleep parameters, along with the corresponding methods/formulas used to estimate their values from actigraphic data. When available, we also present normative reference ranges of the sleep parameters for adults and their potential clinical significance. Each sleep parameter is presented in a separate paragraph with the name of the sleep parameter in bold font. Note that the superscript+ at the end of the name of a sleep parameter indicates that only the activity counts (the minute-by-minute wrist movement values) were used to derive the parameter and hence, derivation of the parameter does not involve sleep scores. On other hand, the superscript¥ indicates that activity counts (the minute-by-minute wrist movement values) were first converted into sleep/wake scores (0’s and 1’s) using sleep scoring algorithm prior to deriving the parameter; derivation of the parameter utilizes only the sleep scores. Demonstration of the derivation of each sleep parameter is presented in the supplemental file (Supplementary Tables S5–S11, available at Annals of Work Exposures and Health online).
Time in bed: TIB refers to the duration a participant spent in bed and is derived by subtracting the time the subject went to bed (in bed time) from the time the subject arose (get up time) (Natale et al., 2014). TIB can be properly ascertained from sleep diary data (participants indicate what time they went to bed and arose) or more objectively by pressing the ‘event-marker’ button on the actigraph, when going to bed (in bed time) and getting out of bed (get up time), which will mark these time points (Martin and Hakim, 2011).
Sleep period: The duration of the interval from sleep onset to sleep offset (O–O interval). We defined sleep onset as the starting time point of the first continuous block of at least 20 min of sleep with no more than 1 min of interruption and sleep offset as the last minute the subject was scored asleep before getting out of bed. The length of SLP depends on the criteria used to define sleep onset and offset (see the review under the heading ‘Identification of TIB and SLP’).
Mean activity during TIB (AMEAN) +: AMEAN refers to the average activity score or frequency of wrist movement (ZCM) per minute while the subject is in bed. It is derived by summing the minute-by-minute activity scores during TIB and dividing the sum by duration of TIB in minutes. A ‘good sleep’ during TIB should yield small values for this parameter while ‘disturbed’ sleep will show higher value for this parameter. This parameter is also referred to as mean motor activity (Natale et al., 2014) and does not have a PSG equivalent. It is computed as follows:
where n is the number of minutes during TIB, is the activity score at the ith minute during TIB.
Median activity during TIB (AMED) +: AMED refers to the median value of activity score (ZCM) per minute during TIB. Often, the frequency of wrist movement per minute while in bed is positively skewed. Hence, the median may be a better measure of the typical activity score per minute than the mean activity level. Higher values for AMED may indicate ‘disturbed’ sleep while in bed. The median is computed by sorting the activity scores during TIB from smallest to largest and identifying the middle value as follows: AMED = {(n + 1) ÷ 2}th ordered value, where n is the number of minutes during TIB.
Activity standard deviation during TIB (ASD) +: ASD refers to the standard deviation of activity scores during TIB. It is a measure of variability in the activity score during TIB; large values may suggest that a subject is showing ‘erratic’ movement behavior while in bed and smaller values may suggest that the subject is consistent in his/her frequency of movement during TIB (consistent does not mean low frequency of movement). It is calculated as follows:
where n is the number of minutes during TIB, ZCMi is the activity score at the ith minute during TIB, and AMEAN is the mean activity during TIB as defined above.
Sleep minutes during TIB (SMIN) ¥: SMIN refers to the number of minutes asleep during TIB. It is the total number of minutes during TIB that the subject was coded (by the algorithm) asleep. The recommended normal for adults is 7–9 h of sleep per night (Berger et al., 2005). It is calculated as follows: where represents the sleep score for the ith minute during TIB, and n is the number of minutes during TIB.
True sleep minutes (TSMIN): TSMIN refers to the number of minutes asleep during SLP (O–O interval). It is the total number of minutes during SLP) that the subject was coded (by the algorithm) asleep. The recommended normal for adults is 7–9 h of sleep per night (Berger et al., 2005). It is calculated as follows: where represents the sleep score for the ith minute during SLP, and k is the number of minutes during SLP.
Sleep onset latency (SOL) ¥: SOL refers to the number of minutes it took a subject to fall asleep. It is the number of minutes between lying down in bed and actually falling asleep (Berger et al., 2005). Technically, it is the number of minutes from the time the subject reported going to bed (in bed time) to the time the subject was first scored as asleep by the algorithm. Normal limits of SOL for adults are <20 min (Berger et al., 2005). Generally, sleep latency derived using actigraphy is less reliable (de Souza et al., 2003; Lichstein et al., 2006; Blackwell et al., 2008; Martin and Hakim, 2011). Quiet wakefulness (e.g. lying down still) tends to be assessed as sleep leading to a less-accurate estimate of SOL as well as sleep duration and wake minutes (Van De Water et al., 2011). A more accurate estimation of this parameter requires at least 14 days of monitoring (Rowe et al., 2008).
Latency to persistent sleep ¥: LPS refers to the number of minutes from the time the subject went to bed to the start of persistent sleep. We define the start of persistent sleep as the time point of the first continuous block of at least 20 min of sleep with no more than 1 min of wakefulness intervening. It is worth noting that the magnitude of LPS depends on the criterion used for defining onset of persistent sleep (e.g. 5, 10, or 15 min of continuous sleep). Cole and colleagues (1992) report that compared to SOL, LPS showed the highest correlation between actigraph and PSG and hence more reliable than SOL.
Percent sleep (PSLP) ¥: PSLP refers to the proportion of minutes a subject was asleep during TIB (proportion of the TIB spent sleeping). PSLP is derived as follows: , where SMIN is sleep duration while in bed and TIB is time spent in bed. Normal limit for adults is ≥80% (Berger et al., 2005).
Sleep efficiency ¥: SE is a measure that is closely related to PSLP. SE is estimated in similar fashion to PSLP, except that it is defined using data from the SLP (‘O–O’ interval) rather than TIB. Therefore, SE is defined as the percentage of time spent asleep during the SLP (between onset of persistent sleep and sleep offset). SE is derived as follows: , where TSMIN is sleep duration during SLP or O–O interval. Unlike PSLP, the numerator does not include periods of immobility (quite wakefulness) that often occurs after first getting into bed. The criterion used for defining the two time points (sleep onset and sleep offset) of the SLP affects the magnitude of this parameter. Normal limit for adults is ≥80% (Berger et al., 2005).
Sleep episodes ¥: SEP refers to the episodes of continuous sleep during TIB (TIB). It is calculated as the total number of episodes during which the subject was continuously asleep (even for 1 min) while in bed. It is simply the count of instances when the subject was asleep for one or more minutes.
Mean sleep episode (MSEP) ¥: MSEP is the average number of minutes the subject was asleep per episode of sleep. It is derived as follows: .
Long sleep episodes (LSEPs) ¥: LSEP refers to the episodes of long sleep during TIB. An episode of sleep is considered long if it lasts at least 5 min (default option in Action-W software). Thus, LSEP is the total number of instances when the subject was asleep for at least 5 min during TIB. Depending on study objective, the user can choose a different criterion for defining LSEP (e.g. 10, 15 min, etc.).
Longest sleep episode (LGSEP) ¥: LGSEP refers to the duration (in minutes) of the longest sleep episode during TIB.
Wake minutes during TIB (WMIN) ¥: WMIN refers to the number of minutes awake during TIB. It is the total number of minutes that the subject was coded (by the algorithm) awake during TIB. It is calculated as: where represents the sleep score for the ith minute during TIB, and n is the number of minutes during TIB.
Wake after sleep onset ¥: WASO refers to the number of minutes a participant was awake between sleep onset and sleep offset (O–O interval). This parameter is similar to WMIN except that it is defined for the true sleep period rather than the major sleep period (TIB). The criterion used for defining the two time points (sleep onset and sleep offset) affects the estimate of this parameter. The value considered normal in adults is <10% of total sleep minutes or 42 min for a person who sleeps 7 h/night (Berger et al., 2005).
Wake episodes (WEPs) ¥: WEP refers to the episodes of awakenings during TIB. It is the count of instances when the subject woke up (for 1 or more minutes) during TIB. In the sleep literature, WEPs are often referred to as number of awakenings. On average, normal values of WEP in adults range from 2 to 6 awakenings per night (Berger et al., 2005). At times, this sleep parameter is defined for the O–O interval, rather than for TIB, which will then represent the number of awakenings between sleep onset and sleep offset.
Mean wake episode (MWEP) ¥: MWEP refers to the average number of minutes the subject was awake per episode of awakening during TIB. It is derived as follows: . In other words, this variable refers to how long, on average, it takes for subject to fall back to sleep after waking up.
Long wake episodes (LWEPs) ¥: LWEP refers to the episodes of long awakenings during TIB. An episode of awakening is considered long if it lasts at least 5 min (default option in Action-W software). It is the count of instances when the subject woke up for at least 5 min during TIB. While the user can choose a different criterion for defining LWEP (e.g. 10, 15 min, etc.), previous studies suggest a cut point of 5 min may have clinical utility. For example, a study (Natale et al., 2009) showed that the number of night awakenings longer than 5 min was one of the key variables that discriminated subjects with insomnia from control subjects. In a population-based study (Tranah et al., 2010), women who currently use hormone therapy had fewer LWEPs (≥5 min) compared to never users.
Longest wake episode (LGWEP) ¥: LGWEP refers to the duration (in minutes) of the longest wake episode during TIB.
Acceleration index (ACCX) +: ACCX refers to the distribution of frequency of movement during TIB. It ranges from −1 to +1, with zero representing uniform distribution of movement. A negative value indicates slowing of activity during TIB while a positive value indicates an acceleration of activity (movement) during TIB (Wiggs and Stores, 2004). The acceleration index (AI) is calculated by the formula: , where p is the proportion of the interval (TIB) required for 50% of the total activity in the interval to be completed. This parameter does not have a clear meaning (Wiggs and Stores, 2004).
Activity index (ACTX) +: ACTX refers to the proportion of minutes during TIB where the activity score was greater than zero. The numerator is count of minutes during TIB where the movement score was greater than zero and the denominator is the total number of minutes during TIB. A very good sleep (no movement at all) yields a zero activity score for each minute and hence a high percentage for this parameter implies a ‘not so good sleep’ (subject showed movement during the majority of TIB regardless of sleep/wake state). A high activity index is indicative of restlessness.
Sleep fragmentation index ¥: SFX refers to the ratio of the number of awakenings to the total sleep time in minutes, during TIB. The numerator is the total number of awakenings during TIB (WEP) while the denominator is the total minutes asleep during TIB (SMIN). SFI is an indicator of restlessness or nocturnal movement (Natale et al., 2014). It is computed as .
Brief wake ratio ¥: BWR refers to the ratio of the number of awakenings lasting only 1 min to the total number of awakenings during TIB. It is the proportion of awakenings that lasted only 1 min. The numerator is count of awakenings during TIB that lasted only 1 min and the denominator is count of all awakenings during TIB. A BWR of zero means there are no awakenings lasting only 1 min (i.e. all awakenings were of >1-min duration) whereas a BWR of 1 means that all awakenings were for just 1 min.
Short burst inactivity index +: SBIX refers to the ratio of the count of zero activity (ZCM = 0) episodes during TIB lasting only 1 min to the count of zero activity episodes during TIB lasting 1 or more minutes. The numerator is the count of minutes during TIB where the subject had a zero-activity score (ZCM = 0) for that minute but with positive activity scores during the preceding and following minutes. The denominator is the count of episodes during TIB where the subject had a zero-activity score for 1 or more minutes. An SBIX = 0 means there are no episodes during TIB where activity score was zero for just 1 min. An SBIX = 100% means all episodes where activity score was zero were for just 1 min (i.e. all episodes of zero activity score had a duration of 1 min).
Measures of intraindividual variability: Although one of the advantages of actigraphy is the ability to collect data for extended period spanning several days or weeks, the focus of most sleep studies involving actigraphy has been on the mean values of the sleep parameters (i.e. average of the sleep measures across days) with little attention to their day-to-day fluctuation (i.e. intraindividual variability or within-subject variability). Intraindividual variability of a sleep parameter is a measure of the day-to-day variability of the sleep parameter and can be computed by calculating the standard deviation of the sleep parameter across the sampling days. We recommend at least 7 days (i.e. seven 24-h periods) of actigraphy data in order to compute a stable measure of daily variability of a sleep parameter. For example, intraindividual variability in sleep duration can be estimated by calculating the standard deviation of sleep minutes () across the sampling days as follows:
where is the standard deviation of sleep minutes () across sampling days, d is the number of sampling days (d ≥ 7), is the sleep minutes on the ith day, and is the average of sleep minutes () across the d sampling days . This parameter, , could provide insight on consistency of sleep duration of a participant across days (i.e. is the sleep duration of the individual consistent from day to day or is sleep duration of the individual erratic with short sleep duration in some days and long sleep duration on other days). The same procedure (formula) can be used to compute measure of intraindividual variability for all the other sleep parameters and this new measure could serve either as predictor or outcome variable in sleep research. For example, a study by Mezick et al. (2009) used within-subject variability in sleep duration and sleep fragmentation as outcome measures and identified psychosocial and physiological stress as potential risk factors for increased nightly variability in these sleep parameters.
Sleep measures that combine mean and variability: Researchers can create new measures by combining the two components of a sleep measure (overall mean and day-to-day variability). For example, using mean sleep duration () and the day-to-day variability associated with sleep duration (), participants could be classified into the following four groups: short and consistent sleepers, long and consistent sleepers, short and variable sleepers, and long and variable sleepers. This can be done for each sleep parameter.
Chronotype: Actigraphic data can also be useful for assessing chronotype, an individual’s preferred time for sleeping (Adan et al., 2012) and classify individuals as early risers versus night owls (Natale et al., 2014). Chronotype is an important sleep parameter to consider as it has been linked to various aspects of health including mental (Taylor and Hasler, 2018) and cardiometabolic outcomes. For example, late chronotypes prefer to wake up later in the morning and go to sleep later in the evening while early chronotypes prefer to wake up early in the morning and go to sleep earlier in the evening. Chronotype is often quantified by calculating the midpoint between the start and end of SLP (Simpkin et al., 2014; de Souza and Hidalgo, 2015; Santisteban et al., 2018; Refinetti, 2019) or midpoint of TIB (Urbanek et al., 2018). The midpoint of SLP or TIB will serve as an estimate of chronotype when examining association of chronotype with psychological or physical well-being (e.g. are individuals with later midpoints at higher risk of poor health outcomes?).
Demonstration of the methodology in actigraphy
In this section, we present demonstration of the methodology involved in actigraphy including: (i) detailed description of the sample actigraphic data with graphical visualization of sleep/wake patterns, (ii) application of the Cole–Kripke algorithm for sleep scoring, (iii) application of sleep rescoring rules, (iv) illustration of TIB versus SLP, and (v) demonstration of the methods for derivation of the sleep parameters.
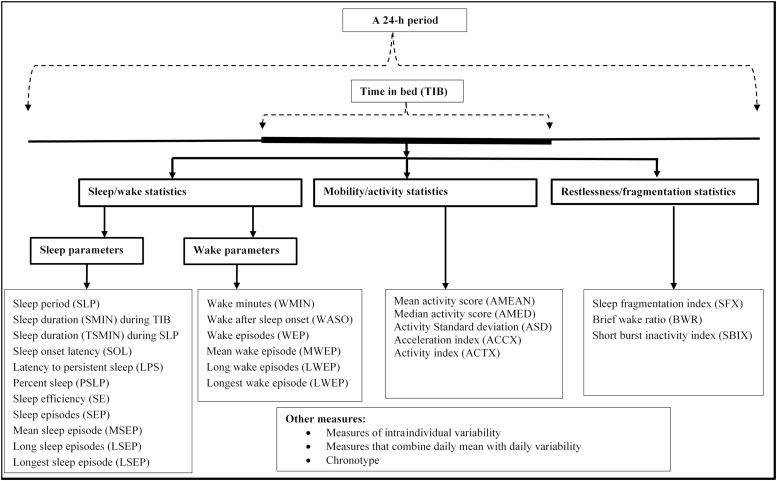
In order to describe/demonstrate the methodologies involved in derivation of sleep parameters from actigraphic data, we obtained wrist movement data collected using the Motionlogger Sleep Watch and analyzed the data using the Action-W software (AMI, Ardsley, New York). The software automatically scores sleep/wake cycles and then estimates sleep parameters for each 24-h period (i.e. a day), as well as the overall average and standard deviation of each parameter based on all days. We then imported the raw wrist movement data into a standard EXCEL spreadsheet where we manually reproduced the sleep parameters estimated by the software; this process aided us in detailing the rules and methods used to derive each sleep measure, and in providing clearer technical definitions of the sleep parameters. The list and type/classification of the sleep parameters that were derived from the actigraphic data is shown in Fig. 2.
Figure 2.
The list and classification of sleep parameters derived from wrist-worn actigraphic data.
Prior to derivation of the various sleep parameters, we defined the beginning of TIB (in bed time) as the time point when lying down in bed the first time and the end of the major sleep period (get up time) as the time point when getting out of bed at the end of a SLP. To mark the end points of the true sleep period, we defined sleep onset as the starting time point of the first continuous block of at least 20 min of sleep with no more than 1 min of interruption and sleep offset as the last minute the subject was scored asleep before getting out of bed. The sleep parameters were derived using data from the major sleep period (TIB). The same procedures (methods) apply to derive the parameters for the true sleep period (O–O interval); all one has to do is restrict the actigraphic data to this subinterval.
The data were from a participant who was enrolled in the Buffalo Cardio-Metabolic Occupational Police Stress (BCOPS) study (Violanti et al., 2006). In the BCOPS study, participants were instructed to wear the actigraph on their non-dominant wrist for 15 days and the device was set to record movement data at 1-min intervals. Participants were instructed to press the event-marker button on the actigraph to digitally mark times they went to bed (‘in bed time’—when lying down in bed the first time) and times they got out of bed (‘get up time’—when getting out of bed at the end of a SLP). Participants also kept a sleep diary for each day where they recorded ‘in bed times’, ‘get up times’, and the times the actigraph was removed from the wrist (i.e. non-wear periods or off-wrist periods). The study was approved by the Internal Review Board of the State University of New York at Buffalo, and the National Institute for Occupational Safety and Health (NIOSH) Institutional Review Board (IRB).
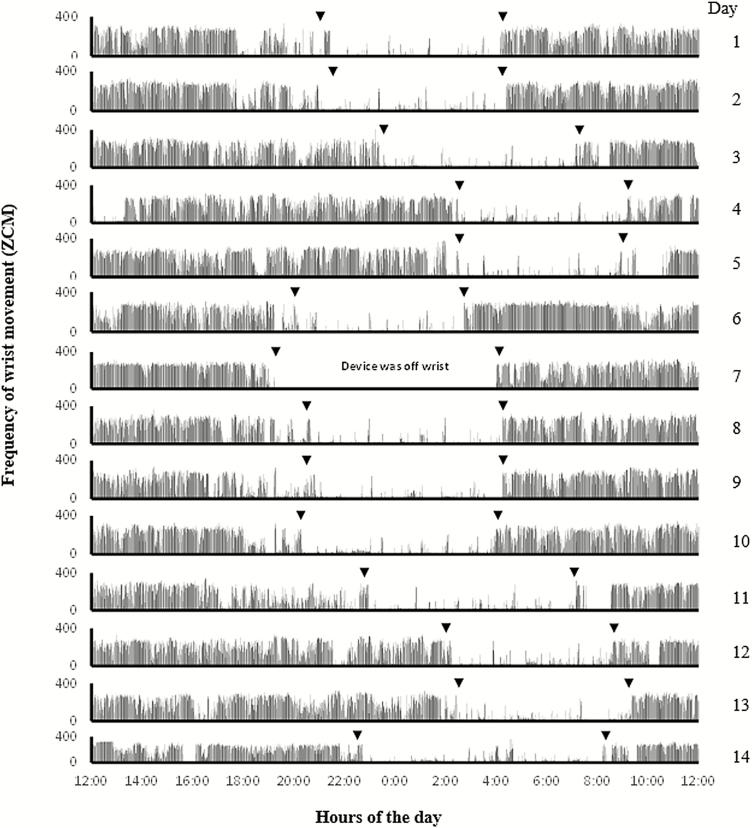
The sample actigraphic data comprise wrist movement data collected in ZCM from one middle-aged participant enrolled in the BCOPS study; data were collected over a 14-day period starting on 11/11/2011 at 12 PM and ending on 11/25/2011 at 11:59 AM. Graphical display of the data for each 24-h period (i.e. from noon to noon on consecutive days) is shown in Fig. 3. The plot displays the minute-by-minute frequency of wrist movement for each day (24-h period); the x-axis represents time of the day in military time (24-h clock) and the y-axis represents the frequency of wrist movement (ZCM). For participants who work on day shift, display of movement data from noon of 1 day to noon of next day (also called the ‘midnight centered view’) is an approach that is recommended for capturing nocturnal sleep behavior and for determining the participant’s habitual bed and waking times (Khawaja et al., 2013). Displaying the data from each day stacked one above the other in one figure (as shown in Fig. 3) also enables the user to visually inspect the sleep/wake patterns simultaneously across multiple days (Martin and Hakim, 2011). From Fig. 3, it appears that there are movement data for 14 days (i.e. 14 24-h periods); however, the participant’s sleep diary indicated that the subject did not wear the actigraph during the night of day #7 (i.e. the device was off wrist from 7:15 PM to 4 AM), as evidenced by the series of zeros (flat line) representing wrist movement values during this period (Fig. 3). Hence, the data from day #7 were excluded, leaving 13 days with movement data for further analysis. The data were analyzed using the Action-W software; the output generated by the software listing the estimated sleep parameters is shown in Supplementary Table S1 (available at Annals of Work Exposures and Health online).
Figure 3.
Actigraphic data of a single participant showing minute-by-minute wrist movement values (activity counts) across 14 days; from 12 PM on 11/11/2011 to 11:59 AM on 11/25/2011. The x-axis represents hours of the day and the y-axis represents the wrist movement values. Each row displays wrist movement for a 24-h period from noon of 1 day to noon of the following day. Downward arrows show ‘in bed times’ and ‘get up times’ for each day ascertained based on information from the event marker and sleep diary. The raw data from the first day is shown in supplemental file (Supplementary Table S2, available at Annals of Work Exposures and Health online) which is used for demonstration of derivation of sleep parameters.
Generally, for a participant with multiple days of actigraphic data, the value of each sleep parameter is calculated separately for each 24-h period (i.e. 1 day) and then the estimates across the multiple days are averaged to get a more stable/representative measure that minimize interdaily variability (Acebo et al., 1999; Tranah et al., 2010). For our demonstration, we present the actual wrist movement data for the first day (first 24-h period) and utilize this data to illustrate derivation of 29 sleep parameters for that particular day. Similar techniques are then applied to estimate the sleep parameters for the remaining 12 days. The raw data for the first day (from 12 PM on 11/11/2011 to 11:59 AM on 11/12/2011) is shown in Supplementary Table S2 (available at Annals of Work Exposures and Health online). On this particular day, based on time stamps from the event marker, the participant went to bed (in bed time) at 21:00 (9 PM) on 11/11/2011 and got out of bed (get up time) at 4:10 AM on 11/12/2011.
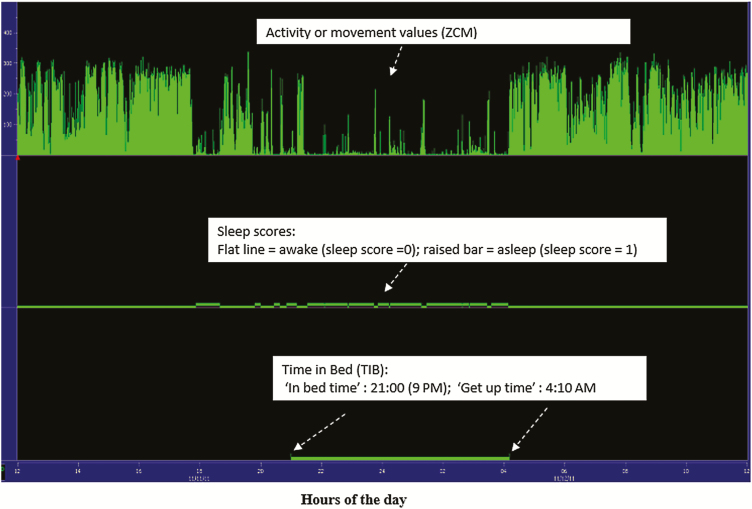
Application of the Cole–Kripke algorithm for assessing sleep/wake cycles (sleep scores) is demonstrated in Supplementary Table S3 (available at Annals of Work Exposures and Health online). Sleep rescoring is demonstrated in Supplementary Table S4 (available at Annals of Work Exposures and Health online). Fig. 4 displays the transformation of the raw wrist movement data (for the first 24-h period) to sleep/wake scores using the Cole–Kripke algorithm. Illustration of the major and true sleep period is shown in Fig. 5. Demonstration of the derivation of sleep parameters for the first 24-h period is shown in Supplementary Tables S5–S11 (available at Annals of Work Exposures and Health online). These illustrations enable readers to have a functional understanding of how the values of each sleep parameter are estimated. Although the demonstration of how to estimate the values of each sleep parameter is provided only for the first 24-h period (first day), the same procedure is then applied to derive the parameters for the remaining 12 days.
Figure 4.
Actigram showing the minute-by-minute wrist movement values (top panel) during the first 24-h period (from 12 PM on 11/11/2011 to 11:59 AM on 11/12/2011), totaling 1440 observations along with the sleep scores estimated using the Cole–Kripke algorithm (middle panel) and TIB ascertained from event marker and sleep diary (bottom panel).
Figure 5.
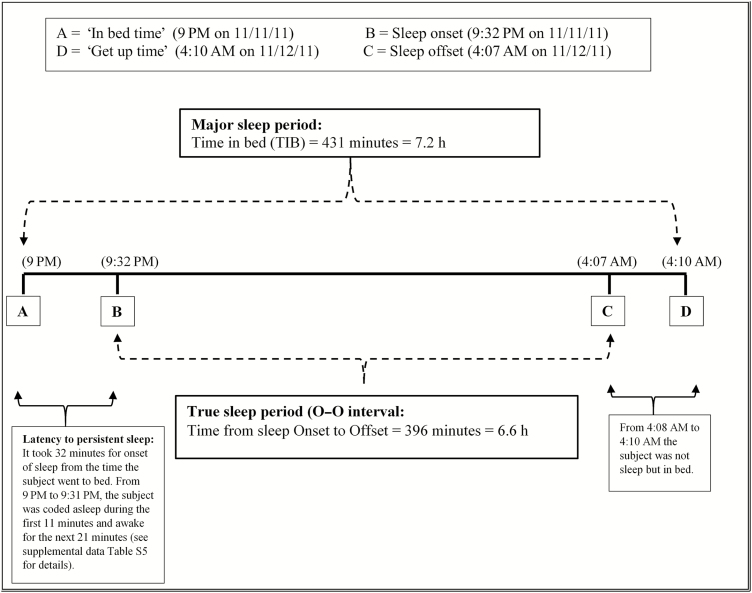
Sketch showing the major sleep period (i.e. TIB) and the true sleep period (i.e. the O–O interval) based on actigraphic data from the first day. The major sleep period is marked by ‘in bed time’ which refers to the time point when first lying down in bed (point A) and ‘get up time’ which refers to the time point when getting out of bed at the end of a SLP (point D). The true sleep period was identified by sleep onset, which was defined as start of the first continuous block of at least 20 min of sleep after first lying in bed (point B), and sleep offset, which was defined as the last minute the subject was scored asleep before getting out of bed (point C). (See Supplementary Table S5, available at Annals of Work Exposures and Health online of supplemental file for further details.)
Discussion
Actigraphic measured wrist activity is considered the most reliable method for objective assessment of sleep in epidemiologic studies and has been validated in a broad range of clinical and non-clinical adult populations (Cole et al., 1992; Lichstein et al., 2006; Blackwell et al., 2008; Tonetti et al., 2008; Rupp and Balkin, 2011; Sadeh, 2011; Van de Water et al., 2011). Despite the wide use of actigraphy in both epidemiologic studies and laboratory settings, publications detailing methods for deriving sleep parameters is sparse. In addition, reporting (i.e. definition and technical details) of actigraphy-based sleep measures (Berger et al., 2005) has been inconsistent across studies (Smith et al., 2018). Discrepancies in how sleep measures are defined could in part reflect differences in both hardware (e.g. sensitivity and specifications of the accelerometer) and software (i.e. sleep parameter criteria) across actigraphic devices (Rupp and Balkin, 2011). In our study, we utilized wrist movement data collected using the Motionlogger actigraph to demonstrate the methodology for estimating sleep parameters. We illustrate how to apply sleep scoring algorithms to translate wrist movement data into sleep scores (sleep/wake cycles) and describe the subsequent steps for deriving common sleep parameters, including: total sleep time, SE, latency, number of awakenings, WASO, and SFX. Emphasis was placed on technical definitions and practical interpretations of the parameters.
Inconsistency in actigraphy-based sleep measures
The most notable inconsistency across studies relates to the algorithms and methods used for identification of sleep onset (i.e. start of sleep) and sleep offset (i.e. end of the last SEP), which mark the SLP or sleep region (see Fig. 5). Some algorithms rely solely on the raw wrist movement values (Sitnick et al., 2008; Natale et al., 2014), while others utilize the sleep/wake scores (de Souza et al., 2003; Tranah et al., 2010) to identify these crucial two time points of the SLP. How these two time points (i.e. sleep onset and sleep offset) are defined ultimately affects the estimates of sleep latency and other sleep measures. For example, sleep latency (the amount of time it takes to fall asleep) has been defined in various ways. These include (i) the interval from bedtime to first occurrence of 10 min block with at least 9 min of no movement (Natale et al., 2014), (ii) the time elapsed from lights out to occurrence of five consecutive sleep minutes (de Souza et al., 2003), (iii) the interval from bedtime to the first occurrence of at least 3 consecutive minutes with activity count of zero (Sitnick et al., 2008), and (iv) the interval from bedtime to start of the first 20-min block with greater than 19 min of sleep (Corkum et al., 2001; Tranah et al., 2010), equivalent to the definition of LPS in this paper. In the current study, SOL was defined as the interval from ‘in bed time’ (when lying down in bed the first time) to the first minute the subject was scored asleep (Action-W software by AMI).
Additional inconsistency across studies relates to the period for which the sleep parameters were reported (i.e. major sleep period versus true sleep period). Some studies (Corkum et al., 2001; Natale et al., 2014) derive sleep parameters (total sleep time, WASO, number of awakenings, and SE) for the true sleep period (O–O interval), while other studies (Tranah et al., 2010) derive sleep measures (sleep hours, SE, WASO, and LWEPs) for the major sleep period (TIB). Lastly, studies often differ in naming conventions for the sleep measures as well as in the formulas used to derive them. For example, Corkum et al. (2001) define restlessness during sleep as the mean frequency of motor movements per minute, which in the current study is labeled as mean activity score during TIB (AMEAN). The sleep measures ‘sleep period (SLP) or O–O interval’, ‘activity index (ACTX)’, and ‘short burst inactivity index (SBIX)’ are named differently by the MotionWare software for Cambridge Actiwatch actigraphs (Cambridge, UK) as ‘assumed sleep period’, ‘mobile time (%)’, and ‘immobile bouts ≤1 min (%)’, respectively. Calculation of specific sleep parameters may also vary by actigraph software. For example, the Action-W software defines the SFX as the ratio of WEPs to total sleep time (in minutes) during TIB (SFX = WEP/SMIN), whereas the MotionWare software calculates the SFX as the sum of ‘mobile time (%)’ and ‘immobile bouts ≤1 min (%)’, which is equivalent to sum of ACTX and SBIX in this study.
In contrast, reporting of TIB has been more consistent, with assessment of bed time (i.e. when first lying down or when lights out) and final wake time (i.e. out of bed or get up time) appearing to be relatively uniform across studies. Nonetheless, the substantial variation observed across studies in the definition of sleep onset and sleep end can render comparison of results challenging, as these measures influence estimates of several key sleep parameters. Hence, researchers need to consider not only the device used and population under study, but also how the sleep parameters of interest are derived or defined, especially when comparing findings across studies (Jean-Louis et al., 2001).
Methodology for deriving sleep measures
A step-by-step demonstration of the methodology presented in this paper provides a basic guide for understanding actigraphic data collection, processing, and interpretation in sleep research, and for the derivation of specific sleep parameters. Understanding how sleep parameters are derived may expand use of sleep measures that are less frequently employed (e.g. day-to-day variability, activity standard deviation, SFX, BWR, and SBIX). For example, depending on the specific objective of a study, one may wish to use standard deviation of activity scores (ASD) during TIB as a predictor of specific health outcomes. Alternatively, within-subject day-to-day variability of the various sleep measures, a measure of intraindividual consistency, may likewise be useful as a predictor or outcome variable (Mezick et al., 2009). In addition, researchers can create new measures by combining the two components of a sleep measure (overall mean and day-to-day variability).
Understanding actigraphy methodology also enables users to modify criteria used for defining certain sleep measures, rather than simply relying on default options provided by the device-specific analysis software. For example, in defining LPS, no more than 1-min of wakefulness is allowed in the first continuous block of at least 20 min of sleep. A researcher may want to permit, for example, a 2- or 3-min period of wakefulness (which is not currently supported by the actigraphy software) and could develop a code to modify the estimation of this parameter accordingly. Similarly, WEP could be redefined as a period of wakefulness lasting at least 5 consecutive minutes rather than the default of 1-min and LWEP could be redefined as a period of wakefulness lasting at least 10 consecutive minutes, rather than the default of 5 min. All three actigraphic devices/software have the ability to export raw actigraphic data into an excel spreadsheet if the user/researcher wishes to redefine sleep parameters using criteria not supported by the software.
In studies examining associations of actigraphy-based sleep parameters (as outcome variables) with other risk factors, statistical models consistent with the nature of the sleep measure ought to be used. Linear regression modeling can be used for sleep parameters that are continuous in nature (e.g. sleep duration), whereas Poisson regression is appropriate for sleep parameters measured as count data (e.g. number of awakenings). In addition, some sleep measures (e.g. sleep hours, efficiency, WASO, LWEPs, etc.) can be categorized using recommended or clinical cut points, which would then allow estimation of prevalence ratios (PR), odds ratios, or risk ratios depending on the study deign (Goyal et al., 2009; Tranah et al., 2010).
Overall, actigraphy tends to overestimate sleep compared to PSG (Van de Water et al., 2011). While rate of agreement between PSG and actigraphy is high in healthy subjects with normal sleep patterns (Sadeh, 2011), agreement rates are lower in those with poor sleep quality, primarily due to the low specificity of the sleep/wake scoring algorithms (as immobile wakefulness is often scored as sleep) (Lichstein et al., 2006). For example, in a 2019 meta-analysis of 96 studies in adults with and without chronic conditions, Conley at al concluded (Conley et al., 2019) that actigraphy overestimated total sleep time (by 11.2 min in healthy adults and by 22.4 min in adults with chronic conditions), and SE (by 1.9% in healthy adults and by 5.2% in those with chronic conditions) compared to PSG, although differences were statistically significant only among those with chronic conditions (Conley et al., 2019). In addition, pooled estimates suggested that actigraphy significantly underestimated SOL compared to PSG in both healthy adults (by 8.1 min) and those with chronic conditions (by 7.7 min); actigraphy-based measures also appeared to underestimate wake time after sleep onset (WASO) relative to PSG, although differences were not significant in either healthy adults or those with chronic conditions. A systematic review and meta-analysis commissioned by the American Academy of Sleep Medicine regarding the clinical utility of actigraphy versus sleep logs and PSG for evaluating a range of sleep disorders yielded findings broadly consistent with those of Conley et al.; specifically, in their review of 81 studies, Smith et al. found substantial evidence that actigrahy underestimates SOL and WASO compared to PSG, and that these differences are clinically meaningful (Smith et al., 2018).
To reduce uncertainty associated with actigraphy it is recommended that actigraphs be used in concert with other objective and subjective methods of sleep assessment when possible (Martin and Hakim, 2011; Sadeh, 2011). It is also important to note that the accuracy of actigraphy versus PSG depends on a number of factors, including the specific sleep variable of interest, the device used, the population under study, the algorithm employed to determine sleep/wake cycles (Van de Water et al., 2011), and the length of the assessment period (Rowe et al., 2008). It is worth noting that defining sleep onset utilizing the raw movement data rather than the sleep scores as in Sitnick et al. (2008) and Natale et al. (2014) may improve accuracy of SOL estimates. Regarding the optimal length of the actigraphic monitoring period, recommendations have varied. Some studies have reported assessment for 5 days or longer to increase reliability and reduce measurement error (Wiggs and Stores, 2004; Sadeh, 2011), while other have indicated means from a 3-day period to be comparable to those obtained from 7 or 14 days (Rowe et al., 2008). However, a minimum monitoring period of 7 days is recommended to address concerns regarding variability of sleep parameters, while a 14-day period is preferable for estimating SOL (Rowe et al., 2008).
Conclusions and directions for future research
This paper presents a detailed demonstration and discussion of the methodology involved in estimating sleep parameters from wrist movement data collected using an actigraphic device (AMI Motionlogger Sleep Watch) commonly used in epidemiologic and clinical studies. Although the methodology presented in this paper is based on a specific actigraphic device and associated sleep/wake algorithms, the overall methodological process is generalizable to other devices and sleep scoring functions. It is critical to recognize that actigraphy does not directly measure sleep (Sadeh, 2011) but rather measures movement, which is then used to estimate sleep/wake cycles. Actigraphy, in essence, involves direct measurement of movement and indirect assessment of sleep through the use of specific algorithms (de Souza et al., 2003; Natale et al., 2014). Therefore, actigraphy-based sleep parameters can be affected by movement disorders and other conditions. Additional limitations include the inability of current sleep scoring algorithms to distinguish between immobile wakefulness and sleep, potentially biasing estimates of certain sleep measures such as latency, total sleep time, and SE (de Souza et al., 2003; Berger et al., 2005; Lichstein et al., 2006; Martin and Hakim, 2011). The search for new and/or improved methodologies and algorithms to more accurately estimate sleep parameters is the focus of ongoing efforts (Sadeh, 2011). Hence, future studies that review and demonstrate methods involved in actigraphy for different devices will expand researchers’ options best suited to their objectives and study populations. Given the range of wrist-activity monitors and analysis software (i.e. scoring algorithms) available for use (Jean-Louis et al., 2001), there is a need to address the methodologic challenges and strengths of the different actigraphic devices used for objective sleep assessment in research. By enhancing overall understanding of the actigraphy process and methodology, the information presented in this paper may help inform the objective assessment of sleep in future research, and improve the decision-making process, interpretation of the derived sleep measures, and the application of these parameters. Finally, there is a need to standardize sleep measures derived from actigraphy in order to facilitate communication among investigators and comparisons across studies. A recent review by a task force of sleep experts (Smith et al., 2018) highlighted the current variability across studies in actigraphy-based sleep measures with respect to algorithms employed, sensitivity threshold settings used, and other factors, underscoring the importance of standardizing actigraphy-based sleep outcomes in future research.
Supplementary Material
Funding
This work was supported by the National Institute for Occupational Safety and Health (NIOSH), contract no. 200-2003-01580. The findings and conclusions in this article are those of the authors and do not necessarily represent the views of the National Institute for Occupational Safety and Health. Mention of company names or products does not imply an endorsement from NIOSH. National Center for Complementary and Integrative Health (grant numbers 1 R15 AT008606 and 3 R15 AT008606-S1).
Conflict of Interest
The authors declare no conflict of interest relating to the material presented in this article.
References
- Acebo C, Sadeh A, Seifer R et al. (1999). Estimating sleep patterns with activity monitoring in children and adolescents: how many nights are necessary for reliable measures? Sleep; 22: 95–103. [DOI] [PubMed] [Google Scholar]
- Action-W User’s Guide, Version 2.7.1 (2011). Ardsley, NY: Ambulatory Monitoring, Inc. [Google Scholar]
- Adan A, Archer SN, Hidalgo MP et al. (2012). Circadian typology: a comprehensive review. Chronobiol Int; 29: 1153–75. [DOI] [PubMed] [Google Scholar]
- Ancoli Israel S, Cole R, Alessi C et al. (2003). The role of actigraphy in the study of sleep and circadian rhythms. Sleep; 26: 342–92. [DOI] [PubMed] [Google Scholar]
- Bellone GJ, Plano SA, Cardinali DP et al. (2016). Comparative analysis of actigraphy performance in healthy young subjects. Sleep Sci, 9: 272–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger AM, Farr LA, Kuhn BR et al. (2007). Values of Sleep/Wake, Activity/Rest, circadian rhythms, and fatigue prior to adjuvant breast cancer chemotherapy. J Pain Symptom Manag; 33: 398–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger AM, Parker KP, Young-McCaughan S et al. (2005) Sleep wake disturbances in people with cancer and their caregivers: state of the science. Oncol Nurs Forum; 32: E98–126. [DOI] [PubMed] [Google Scholar]
- Blackwell T, Redline S, Ancoli-Israel S et al. ; Study of Osteoporotic Fractures Research Group. (2008) Comparison of sleep parameters from actigraphy and polysomnography in older women: the SOF study. Sleep; 31: 283–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CamNtech (2018). The MotionWatch 8 and MotionWare User Guide. Available at http://www.medicoimpianti.it/files/The-MotionWatch-User-Guide.pdf. Accessed 3 November 2019.
- Cole RJ, Kripke DF, Gruen W et al. (1992). Automatic sleep/wake identification from wrist activity. Sleep; 15: 461–9. [DOI] [PubMed] [Google Scholar]
- Conley S, Knies A, Batten J et al. (2019) Agreement between actigraphic and polysomnographic measures of sleep in adults with and without chronic conditions: a systematic review and meta-analysis. Sleep Med Rev; 46: 151–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corkum P, Tannock R, Moldofsky H et al. (2001). Actigraphy and parental ratings of sleep in children with attention-deficit/hyperactivity disorder (ADHD). Sleep; 24: 303–12. [DOI] [PubMed] [Google Scholar]
- de Souza L, Benedito-Silva AA, Pires ML et al. (2003). Further validation of actigraphy for sleep studies. Sleep; 26: 81–5. [DOI] [PubMed] [Google Scholar]
- de Souza CM, Hidalgo MP (2015). The midpoint of sleep on working days: a measure for chronodisruption and its association to individuals’ well-being. Chronobiol Int; 2: 341–8. [DOI] [PubMed] [Google Scholar]
- Fawkes DB, Malow BA, Weiss SK et al. (2015). Conducting actigraphy research in children with neurodevelopmental disorders—a practical approach. Behav Sleep Med; 13: 181–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal D, Gay C, Lee K (2009). Fragmented maternal sleep is more strongly correlated with depressive symptoms than infant temperament at three months postpartum. Arch Women Ment Health; 12: 229–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hersen M.(2006). Clinician’s handbook of child behavioral assessment. Burlington, MA: Elsevier. [Google Scholar]
- Jean-Louis G, Kripke DF, Mason WJ et al. (2001). Sleep estimation from wrist movement quantified by different actigraphic modalities. J Neurosci Methods; 105: 185–91. [DOI] [PubMed] [Google Scholar]
- Jean-Louis G, Von Gizycki H, Zizi F et al. (1997a). The actigraph data analysis software: I. A novel approach to scoring and interpreting sleep-wake activity. Percept Motor Skills; 85: 207–16. [DOI] [PubMed] [Google Scholar]
- Jean-Louis G, Von Gizycki H, Zizi F et al. (1997b). The actigraph data analysis software: II. A novel approach to scoring and interpreting sleep-wake activity. Percept Motor Skills; 85: 219–26. [DOI] [PubMed] [Google Scholar]
- Khawaja IS, M Hashmi A, Westermeyer J et al. (2013) Nocturnal awakening & sleep duration in veterans with PTSD: an actigraphic study. Pak J Med Sci; 29: 991–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichstein KL, Stone KC, Donaldson J et al. (2006). Actigraphy validation with insomnia. Sleep; 29: 232–9. [PubMed] [Google Scholar]
- Littner M, Kushida CA, Anderson WM et al. ; Standards of Practice Committee of the American Academy of Sleep Medicine. (2003) Practice parameters for the role of actigraphy in the study of sleep and circadian rhythms: an update for 2002. Sleep; 26: 337–41. [DOI] [PubMed] [Google Scholar]
- Martin JL, Hakim AD (2011) Wrist actigraphy. Chest; 139: 1514–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer LJ, Montgomery-Downs HE, Insana SP et al. (2012a). Use of actigraphy for assessment in pediatric sleep research. Sleep Med Rev; 16: 463–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer LJ, Walsh CM, Traylor J et al. (2012b). Direct comparison of two new actigraphs and polysomnography in children and adolescents. Sleep; 35: 159–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezick EJ, Matthews KA, Hall M et al. (2009). Intra-individual variability in sleep duration and fragmentation: associations with stress. Psychoneuroendocrinology; 34: 1346–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natale V, Léger D, Martoni M et al. (2014). The role of actigraphy in the assessment of primary insomnia: a retrospective study. Sleep Med; 15: 111–5. [DOI] [PubMed] [Google Scholar]
- Natale V, Plazzi G, Martoni M (2009) Actigraphy in the assessment of insomnia: a quantitative approach. Sleep; 32: 767–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Refinetti R. (2019) Chronotype variability and patterns of light exposure of a large cohort of United States residents. Yale J Biol Med; 92: 179–86. [PMC free article] [PubMed] [Google Scholar]
- Respironics (2018). Actiwatch Communication and Sleep Analysis Software. Instruction manual. Available at https://johnawinegarden.files.wordpress.com/2015/03/actiwatchsoftware.pdf. Accessed 3 November 2019.
- Rowe M, McCrae C, Campbell J et al. (2008). Actigraphy in older adults: comparison of means and variability of three different aggregates of measurement. Behav Sleep Med; 6: 127–45. [DOI] [PubMed] [Google Scholar]
- Rupp TL, Balkin TJ (2011). Comparison of Motionlogger Watch and Actiwatch actigraphs to polysomnography for sleep/wake estimation in healthy young adults. Behav Res Methods; 43: 1152–60. [DOI] [PubMed] [Google Scholar]
- Sadeh A. (2011). The role and validity of actigraphy in sleep medicine: an update. Sleep Med Rev; 15: 259–67. [DOI] [PubMed] [Google Scholar]
- Sadeh A, Sharkey KM, Carskadon MA (1994). Activity-based sleep-wake identification: an empirical test of methodological issues. Sleep, 17: 201–7. [DOI] [PubMed] [Google Scholar]
- Santisteban JA, Brown TG, Gruber R (2018). Association between the Munich Chronotype Questionnaire and Wrist Actigraphy. Sleep Disord; 2018: 5646848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpkin CT, Jenni OG, Carskadon MA et al. (2014). Chronotype is associated with the timing of the circadian clock and sleep in toddlers. J Sleep Res; 23: 397–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitnick SL, Goodlin-Jones BL, Anders TF (2008) The use of actigraphy to study sleep disorders in preschoolers: some concerns about detection of nighttime awakenings. Sleep; 31: 395–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MT, McCrae CS, Cheung J et al. (2018) Use of actigraphy for the evaluation of sleep disorders and circadian rhythm sleep-wake disorders: an American Academy of Sleep Medicine Systematic Review, meta-analysis, and GRADE assessment. J Clin Sleep Med; 14: 1209–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor BJ, Hasler BP (2018). Chronotype and mental health: recent advances. Curr Psychiatry; 20: 59. [DOI] [PubMed] [Google Scholar]
- Tonetti L, Pasquini F, Fabbri M et al. (2008). Comparison of two different actigraphs with polysomnography in healthy young subjects. Chronobiol Int; 25: 145–53. [DOI] [PubMed] [Google Scholar]
- Tranah GJ, Parimi N, Blackwell T et al. (2010) Postmenopausal hormones and sleep quality in the elderly: a population based study. BMC Womens Health; 10: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbanek JK, Spira AP, Di J et al. (2018). Epidemiology of objectively measured bedtime and chronotype in US adolescents and adults: NHANES 2003–2006. Chronobiol Int; 35: 416–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- User’s Guide to Action4, Version 1.1 (2010). Ardsley, NY: Ambulatory Monitoring, Inc. [Google Scholar]
- Van de Water AT, Holmes A, Hurley DA (2011). Objective measurements of sleep for non-laboratory settings as alternatives to polysomnography—a systematic review. J Sleep Res; 20(1 Pt 2): 183–200. [DOI] [PubMed] [Google Scholar]
- Violanti JM, Burchfiel CM, Miller DB et al. (2006). The buffalo cardio-metabolic occupational police stress (BCOPS) pilot study: methods and participant characteristics. Ann Epidemiol; 16: 148–56. [DOI] [PubMed] [Google Scholar]
- Webster JB, Kripke DF, Messin S et al. (1982) An activity-based sleep monitor system for ambulatory use. Sleep; 5: 389–99. [DOI] [PubMed] [Google Scholar]
- Wiggs L, Stores G (2004). Sleep patterns and sleep disorders in children with autistic spectrum disorders: insights using parent report and actigraphy. Dev Med Child Neurol; 46: 372–80. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.