Significance Statement
The secretion of organic solutes by the proximal tubules is an essential intrinsic kidney function. However, the clinical significance of the kidney clearance of tubular secretory solutes is uncertain. In this prospective cohort study of 3416 participants with CKD, the authors found that lower kidney clearances of six endogenous secretory solutes are associated with significantly greater risk of CKD progression (defined as a ≥50% decline in eGFR from baseline, initiation of maintenance dialysis, or kidney transplantation) and that lower clearances of four solutes are associated with all-cause mortality after adjustment for eGFR, albuminuria, and other confounding characteristics. These findings suggest that kidney clearances of secretory solutes may provide complementary information to existing measurements of GFR and albuminuria for the assessment of kidney health.
Keywords: proximal tubular secretion, secretory solutes clearances, CKD progression, mortality
Visual Abstract
Abstract
Background
The secretion of organic solutes by the proximal tubules is an essential intrinsic kidney function. However, the clinical significance of the kidney’s clearance of tubular secretory solutes is uncertain.
Methods
In this prospective cohort study, we evaluated 3416 participants with CKD from the Chronic Renal Insufficiency Cohort (CRIC) study. We measured plasma and 24-hour urine concentrations of endogenous candidate secretory solutes at baseline, using targeted liquid chromatography–tandem mass spectrometry. The study defined CKD progression by a ≥50% decline in the eGFR, initiation of maintenance dialysis, or kidney transplantation. We used Cox proportional hazards regression to test associations of secretory-solute clearances with CKD progression and mortality, adjusting for eGFR, albuminuria, and other confounding characteristics.
Results
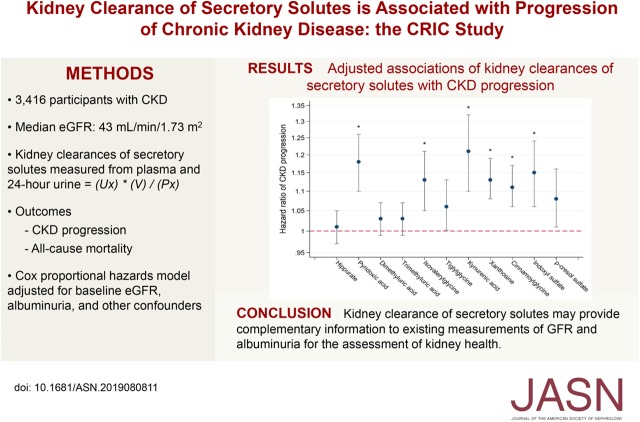
Participants in this ancillary study had a mean age of 58 years and 41% were black; the median eGFR was 43 ml/min per 1.73 m2. After adjustment, lower kidney clearances of six solutes—kynurenic acid, pyridoxic acid, indoxyl sulfate, xanthosine, isovalerylglycine, and cinnamoylglycine—were associated with significantly greater risks of CKD progression, with clearance of kynurenic acid, a highly protein-bound solute, having the strongest association. Lower clearances of isovalerylglycine, tiglylglycine, hippurate, and trimethyluric acid were significantly associated with all-cause mortality after adjustment.
Conclusions
We found lower kidney clearances of endogenous secretory solutes to be associated with CKD progression and all-cause mortality, independent of eGFR and albuminuria. This suggests that tubular clearance of secretory solutes provides additional information about kidney health beyond measurements of glomerular function alone.
CKD affects approximately 30 million American adults.1 Current strategies for detecting and monitoring CKD focus on the measurement of glomerular functions; specifically, the presence and stage of CKD are defined by the GFR and urinary albumin excretion.2 However, the kidneys perform many vital homeostatic functions outside of the glomerular compartment, including secretion of organic solutes and drugs, synthesis of vital hormones and amino acids, and maintenance of acid-base and salt-water homeostasis.3–5 Developing reliable measurements to evaluate these nonglomerular functions could advance our understanding of kidney health.
The kidneys eliminate retained solutes via both glomerular filtration and tubular secretion. Filtration is the primary mechanism for removing freely circulating, low molecular weight solutes. In parallel, tubular secretion involves the direct extraction of solutes from the circulation via specialized transporters on proximal tubular epithelial cells and extrusion of these solutes into the urine against an energy gradient.6,7 The secretion of organic solutes parallels renal blood flow and requires active and coordinated processes, including mitochondrial respiration, energy-coupled transport, and maintenance of cell polarity, suggesting possible contrasts with GFR. Consequently, the clearance of secretory solutes may capture information about the severity of kidney disease that is not revealed by measurements of glomerular filtration and integrity alone.
Tubular secretory solute clearance is rarely measured or estimated due to uncertainty regarding optimal endogenous solutes and nonstandardized laboratory assays. We developed a targeted assay to measure specific secretory solutes in blood and urine, and we used this assay to quantify solutes in 24-hour urine and paired plasma samples from a national cohort study of CKD. We hypothesized that lower kidney clearances of endogenous secretory solutes are associated with CKD progression and mortality independent of estimated GFR and albuminuria.
Methods
Data Source and Participants
We performed an ancillary study to the Chronic Renal Insufficiency Cohort (CRIC) study, a prospective study of CKD progression and cardiovascular disease among patients with CKD.8,9 From 2003 to 2008, the CRIC study originally enrolled 3939 patients with an eGFR of between 20 and 70 ml/min per 1.73 m2 for ages 21–44 years, 20 and 60 ml/min per 1.73 m2 for ages 45–64 years, and 20 and 50 ml/min per 1.73 m2 for ages 65–74 years. Major exclusion criteria included kidney transplantation, active immunosuppression, polycystic kidney disease, multiple myeloma, pregnancy, HIV infection, cirrhosis, and severe heart failure. Study participants completed annual in-person follow-up visits and were contacted by telephone at 6-month intervals.
For the purposes of this ancillary study, we excluded 515 participants who did not have 24-hour urine or plasma samples available from the baseline CRIC study visit and eight participants who had a baseline eGFR of <15 ml/min per 1.73 m2 based on the CRIC study equation. For analyses of CKD progression, we further excluded 209 participants who lacked follow-up data for this outcome (Supplemental Figure 1). The CRIC protocol was approved by institutional review boards at all participating institutions. All participants provided written informed consent.
Measurements of Kidney Clearances of Suspected Secretory Solutes
Through literature review, we selected candidate secretory solutes based on one or more of the following characteristics: reported specificity for organic anion transporters (OAT1 and OAT3), an increase in circulating concentrations after OAT3-transporter knockout in experimental models, a high reported protein-binding percentage, and/or kidney clearances that substantially exceed GFR or creatinine clearance.10–12 We then developed a targeted liquid chromatography–tandem mass spectrometry (LC-MS/MS) assay for these solutes in plasma and urine.13 We determined protein-binding characteristics for each solute in patients with CKD using centrifugal ultrafiltration (Table 1). These studies revealed lower protein-binding percentages than previously reported for three solutes (isovalerylglycine, tiglylglycine, and xanthosine). However, we retained these solutes in our analyses because their kidney clearances greatly exceeded eGFR, suggesting tubular secretion as the major kidney pathway of elimination.
Table 1.
Kidney clearances of secretory solutes and correlations with eGFR
| Solute | Kidney Clearance, ml/min (IQR)a | Correlation with eGFRb,c | Protein Binding in CKD, %d |
|---|---|---|---|
| Hippurate | 465 (274, 807) | 0.33 | 51±13 |
| Pyridoxic acid | 456 (284, 716) | 0.56 | 87±1 |
| Dimethyluric acid | 452 (258, 804) | 0.35 | 68±7 |
| Trimethyluric acid | 260 (134, 499) | 0.38 | 80±11 |
| Isovalerylglycine | 225 (142, 348) | 0.50 | 4±7 |
| Tiglylglycine | 182 (111, 286) | 0.56 | 24±15 |
| Kynurenic acid | 92 (63, 136) | 0.60 | 96±2 |
| Xanthosine | 77 (46, 121) | 0.44 | 15±13 |
| Cinnamoylglycine | 57 (33, 101) | 0.39 | 95±3 |
| Indoxyl sulfate | 34 (22, 51) | 0.55 | 93±2 |
| p-Cresol sulfate | 10 (6, 15) | 0.52 | 96±1 |
Median (IQR) of kidney clearances (not standardized for body surface area).
Pearson correlations between log-transformed secretory-solute clearances and log-transformed eGFR, each standardized to 1.73 m2 body surface area.
P values for all correlations <0.001.
Shown as mean±SD. To evaluate the extent of protein binding, we performed ultrafiltration experiments in plasma obtained from 14 patients with advanced CKD not receiving dialysis. Briefly, plasma was filtered using a centrifugal filter (Amicon Ultra, 3 kD MWCO) at 11,200×g for 30 min at room temperature. The concentration of solutes in the filtrate was then determined using the same method as for plasma and compared with the concentration of solutes in unfiltered plasma. The proportion of the solutes in the filtrate was assumed to be unbound in plasma. Preliminary experiments were performed to confirm that observed differences between the filtrate and unfiltered plasma could not be attributed to adsorption to the Amicon Ultra filtration units (data not shown).
We measured total plasma concentrations of secretory solutes at the CRIC baseline visit using protein precipitation, solid phase extraction, and LC-MS/MS, and we measured 24-hour urine concentrations at the baseline visit using solid phase extraction and LC-MS/MS. Calibration was achieved using a single-point calibration approach to account for potential drift that may be caused by changes in reagents, calibrator lots, equipment, or settings. First, the peak areas of each solute were normalized to peak areas of labeled internal standards added to each well. Next, peak area ratios were standardized to the single-point calibrators (set of five replicates on each study plate). Laboratory coefficients of variation were generally low (Supplemental Table 1). We have previously determined accurate concentrations of each secretory solute in the single-point calibrators (pooled human serum and urine) by standard addition of solutions of pure compounds analyzed by quantitative nuclear magnetic resonance.
We calculated the clearance of each solute as:
where UX represents the 24-hour urine concentration of solute X, V represents the 24-hour urine volume, and PX represents the plasma concentration. We focused on the kidney clearances of suspected secretory solutes, rather than plasma levels alone, to avoid the potential influence of nonkidney-related factors on circulating levels.
Measurement of Outcomes
Study outcomes were CKD progression and all-cause mortality. The CRIC study defined CKD progression by a 50% decline in eGFR from baseline, the initiation of chronic dialysis, or kidney transplantation, whichever occurred first. We also evaluated the slope of GFR decline during follow-up as a secondary outcome. GFR was estimated at each CRIC study visit based on concentrations of serum creatinine and cystatin C, age, sex, and black race using an equation that was developed in a subcohort of 1433 CRIC participants who underwent 125-iothalamate GFR (iGFR) clearance studies.14 Serum-creatinine concentrations were measured using an enzyme-based assay on the Hitachi Vitros 950 AT with values traceable to isotope dilution mass spectrometry. Serum cystatin-C concentrations were measured using a Siemens BNII instrument and longitudinal control materials were used to correct for drift over time when using different calibrator and reagent lots.15 Ascertainment of dialysis and transplantation status in the CRIC study was based on self-report during biannual surveillance and supplemented by crosslinkage with the United States Renal Data System. Mortality was ascertained from reports of next of kin, death certificates, hospital records, and linkage with the Social Security Death Master File.16 To address the potential issue of competing risk, we also evaluated a composite end point of CKD progression or all-cause death.17
Measurements of Covariates
Trained study staff collected participants’ self-reported baseline information regarding sociodemographic characteristics, medical histories, and current medications.8,9 Serum albumin, phosphorus, and glucose were measured on the Hitachi Vitros 950 AT. iGFR was measured in a subset of 1433 participants at baseline using a standardized protocol after a low protein (<10 g) meal. Urinary albumin was measured on a Siemens Immulite.14 Resting seated BP was measured three times, and the average of the second and third readings recorded. Diabetes was defined by a fasting glucose concentration ≥126 mg/dl (7.0 mmol/L), a nonfasting glucose ≥200 mg/dl (11.1 mmol/L), or the use of antidiabetic medications. The cause of CKD was self-reported at baseline.
Statistical Analyses
We created a summary measure of the clearances of secretory solutes to facilitate the tabular presentation of participant characteristics and to provide a singular metric for summarizing associations with study outcomes. Because the kidney clearances of each solute inherently reside on different scales, we log transformed the individual clearances and then standardized them to a common 0–100 scale:
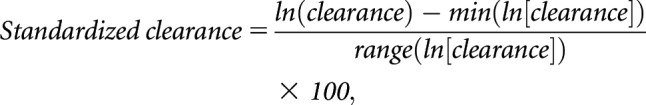 |
where ln(clearance) represents the natural log-transformed clearance value, min(ln[clearance]) represents the minimum value of ln-clearance in the distribution, and range(ln[clearance]) represents the difference between the maximum and minimum values. We then computed the summary score as the average of the 11 standardized clearances (Supplemental Figure 2). This computed summary score is specific to the CRIC study population and may obscure potential contrasts among the individual solutes. To identify potential characteristics associated with the summary secretion score independent of eGFR, we regressed this score on each baseline characteristic adjusting for eGFR only, or for eGFR plus age, sex, race, and body mass index (BMI).
We used Cox proportional hazards regression to estimate associations of kidney clearances of suspected secretory solutes at baseline with study outcomes. Primary models of CKD progression were censored for mortality; secondary models considered a composite outcome of CKD progression or death. The exact time of halving of eGFR was interpolated assuming a linear decline between study visits.18 We constructed nested models to evaluate potential confounding characteristics. Model 1 adjusted for age, race, sex, baseline eGFR, and log-transformed 24-hour urinary albumin excretion. Model 2 additionally adjusted for clinical site, BMI, history of diabetes, smoking status, systolic BP, history of cardiovascular disease, and use of angiotensin-converting enzyme inhibitors or angiotensin receptor blockers. We determined that 1206 events of CKD progression in a total analytic sample size of 3207 participants would provide at least 90% power to detect a hazard ratio of 1.13 per 10-units-lower summary secretion score (SD=7.6) at a two-sided α level of 0.05. Given correlations among the clearances of secretory solutes, we did not mutually adjust for multiple clearances in the same model. We investigated the possibility that baseline levels of eGFR or albuminuria could modify associations between clearances of secretory solutes and rates of study outcomes using stratified Poisson regression.
For GFR slope analyses, we used linear mixed-effects models with both random intercepts and random slopes to assess the association between kidney clearances of secretory solute at baseline and the change in eGFR, adjusting for the same covariates as in the above survival analysis. Natural log-transformed values of eGFR at each study visit served as the dependent variable and differences in slope were assessed by a time-by-exposure interaction. The results of these models can be interpreted as the difference in annualized decline of eGFR per 50% lower in the kidney clearance of each secretory solute.
In sensitivity analyses, we added adjustment for medications that could potentially influence the clearances of secretory solutes through interactions with basolateral transporters. To address the possibility of residual confounding by GFR, we repeated our analyses among the subsample of participants who completed iGFR measurements at baseline with additional adjustment for iGFR. To test generalizability, we repeated our analyses using GFR estimated by the CKD Epidemiology Collaboration equation, a more widely used equation, to define CKD progression.19 We used the Hommel method to correct for multiple comparisons.20 A two-sided P value of 0.05 after correction was used to declare statistical significance. Because covariates were missing in <1% of participants, we used complete-case analysis. We used scaled Schoenfeld residuals to test the proportional hazards assumption in Cox models. No evidence of violation of the proportional hazard assumption was detected. We estimated changes in the regression coefficients upon deleting each observation in turn to check the possibility of influential observations. Analyses were performed using Stata/IC 14.2 (Stata Statistical Software: release 14; StataCorp, College Station, TX) and RStudio 3.4.3 (R Core Team, Vienna, Austria).
Results
Study Population and Characteristics
Participants included in this ancillary study were characterized by a mean age of 58 ± 11 years, 45% were female, 41% were black, 12% were Hispanic, and median eGFR was 43 ml/min per 1.73 m2 (interquartile range [IQR], 32–55 ml/min per 1.73 m2). The kidney clearances of secretory solutes ranged from a median of 465 ml/min (IQR, 274–807 ml/min) for hippurate to a median of 10 ml/min (IQR, 6–15 ml/min) for p-cresol sulfate (Table 1). The clearances of most secretory solutes were considerably higher than eGFR. For all secretory solutes, higher eGFR was associated with higher kidney clearances and lower plasma concentrations (Supplemental Table 2). Correlations between eGFR and the kidney clearances of secretory solutes ranged from 0.33 for hippurate to 0.60 for kynurenic acid (P values for all correlations <0.001). Correlations among the individual solute clearances ranged from 0.30 to 0.75 (Supplemental Table 3). The summary secretion score, which represents the average of the individual transformed clearances, was higher among participants with higher eGFR and among participants who were younger, male, nonblack, non-Hispanic, with higher BMI, higher education attainment, and a lower prevalence of cardiovascular disease (Table 2). After adjustment, older age; male sex; nonblack race; college-graduate status; higher BMI; higher urinary albumin excretion; a history of diabetes; and the use of insulin, statins, thiazide diuretics, and angiotensin-converting enzyme inhibitors or angiotensin receptor blockers were associated with a higher summary secretion score (Supplemental Table 4). The kidney clearances of secretory solutes tended to be higher among participants who reported GN as the cause of CKD (Supplemental Table 5).
Table 2.
Baseline participant characteristics by quartiles of the summary secretion score
| Characteristics | All Participants | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 |
|---|---|---|---|---|---|
| eGFRCRIC, ml/min per 1.73 m2 | 45±17 | 32±11 | 40±13 | 48±13 | 60±16 |
| Age, yr | 58±11 | 59±11 | 58±11 | 58±11 | 56±10 |
| Female | 1531 (45) | 449 (53) | 427 (50) | 344 (40) | 305 (36) |
| Black | 1410 (41) | 406 (48) | 359 (42) | 322 (38) | 317 (37) |
| Hispanic | 405 (12) | 141 (17) | 112 (13) | 90 (11) | 62 (7) |
| BMI, kg/m2 | 32±8 | 31±8 | 32±8 | 32±7 | 33±8 |
| Education categories | |||||
| Less than high school | 678 (20) | 250 (29) | 191 (22) | 143 (17) | 94 (11) |
| High school graduate | 647 (19) | 186 (22) | 168 (20) | 151 (18) | 142 (17) |
| Some college | 983 (29) | 246 (29) | 238 (28) | 255 (30) | 244 (29) |
| College graduate or higher | 1097 (32) | 169 (20) | 255 (30) | 303 (36) | 370 (44) |
| Current smoker | 435 (13) | 132 (16) | 106 (12) | 99 (12) | 97 (11) |
| History of diabetes | 1637 (48) | 412 (48) | 434 (51) | 421 (49) | 367 (43) |
| History of cardiovascular disease | 1142 (33) | 351 (41) | 302 (35) | 284 (33) | 200 (24) |
| History of peripheral vascular disease | 232 (7) | 87 (10) | 60 (7) | 43 (5) | 42 (5) |
| Systolic BP, mm Hg | 128±22 | 133±25 | 128±22 | 126±20 | 124±19 |
| Laboratory measurementsa | |||||
| Serum albumin, g/dl | 3.9±0.5 | 3.9±0.5 | 3.9±0.5 | 4.0±0.5 | 4.0±0.4 |
| 24-h urine albumin, g/24 h | 0.7+1.7 | 0.8±1.6 | 0.8±1.9 | 0.7±1.6 | 0.5±1.5 |
| Phosphate, mg/dl | 3.7±0.7 | 4.0±0.7 | 3.8±0.6 | 3.6±0.6 | 3.5±0.5 |
| HDL, mg/dl | 48±16 | 47±16 | 48±15 | 47±15 | 48±15 |
| Total bicarbonate, mEq/L | 24.4±3.2 | 23.4±3.4 | 24.3±3.2 | 24.7±3.0 | 25.3±3.0 |
| Hemoglobin, g/dL | 12.6±1.8 | 12.0±1.7 | 12.4±1.6 | 12.8±1.8 | 13.2±1.7 |
| Medications | |||||
| Insulin | 824 (24) | 211 (25) | 233 (28) | 214 (25) | 166 (20) |
| Statin | 1872 (55) | 455 (54) | 483 (57) | 489 (58) | 439 (52) |
| ACEi/ARB | 2323 (68) | 526 (62) | 624 (73) | 624 (73) | 544 (64) |
| Loop diuretic | 1276 (38) | 437 (52) | 363 (43) | 270 (32) | 204 (24) |
| Thiazide diuretic | 966 (28) | 184 (22) | 255 (30) | 281 (33) | 244 (29) |
For continuous variables, mean±SD; for categoric variables, N (%); unless otherwise specified. eGFRCRIC, glomerular filtration rate estimated based on serum creatinine and cystatin C concentrations, age, sex, and Black race using an equation that was developed in a subcohort of 1433 CRIC participants who underwent 125-Iothalamate GFR clearance studies; ACEi, angiotensin-converting enzyme inhibitors; ARB, angiotensin receptor blockers.
Conversion factors for units: albumin in g/dl to g/L, ×10; phosphate in mg/dl to mmol/L, ×0.3229; HDL in mg/dl to mmol/L, ×0.02586; bicarbonate in mEq/L to mmol/L, ×1; hemoglobin in g/dl to g/L, ×10.
Associations of Kidney Clearances of Secretory Solutes with CKD Progression
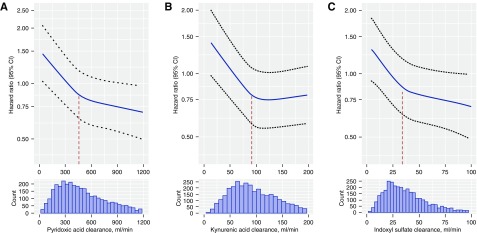
Over a median follow-up of 6.0 years, 1206 participants had CKD progression, defined as a 50% decline in eGFR from baseline, the initiation of chronic dialysis, or kidney transplantation. In unadjusted models, lower kidney clearances of each secretory solute at baseline were associated with greater risks of CKD progression (Table 3). Adjustment for baseline eGFR, albuminuria, and demographics attenuated the size of these observed associations. Further adjustment for other potential confounding characteristics had only a small additional effect on the results. After full adjustment and accounting for multiple comparisons, lower kidney clearances of kynurenic acid, pyridoxic acid, indoxyl sulfate, xanthosine, isovalerylglycine, and cinnamoylglycine were associated with significantly greater risks of CKD progression. Effect sizes ranged from an 11%–21% greater risk per 50% lower clearance. For most associations, hazard ratios for CKD progression tended to follow a curvilinear pattern, with more prominent associations observed at the low to middle ranges of the distributions (Figure 1). When analyzed across categories of eGFR and albuminuria, CKD progression rates were higher for lower quartiles of the summary secretion score (Supplemental Table 6). Contrasts in progression rates across these quartiles tended to be larger for categories of lower albuminuria at baseline.
Table 3.
Associations between kidney clearances of secretory solutes and CKD progression
| Solute | Unadjusted | Model 1a | Model 2b | |||
|---|---|---|---|---|---|---|
| HR (95% CI)c | P Value | HR (95% CI)c | P Value | HR (95% CI)c | P Value | |
| Hippurate | 1.15 (1.11 to 1.18) | <0.001d | 0.99 (0.95 to 1.03) | 0.61 | 1.01 (0.97 to 1.05) | 0.74 |
| Pyridoxic acid | 1.53 (1.46 to 1.60) | <0.001d | 1.18 (1.11 to 1.26) | <0.001d | 1.18 (1.10 to 1.26) | <0.001d |
| Dimethyluric acid | 1.17 (1.13 to 1.22) | <0.001d | 1.03 (0.99 to 1.07) | 0.21 | 1.03 (0.99 to 1.07) | 0.21 |
| Trimethyluric acid | 1.19 (1.15 to 1.23) | <0.001d | 1.03 (0.99 to 1.07) | 0.16 | 1.03 (0.99 to 1.07) | 0.12 |
| Isovalerylglycine | 1.51 (1.43 to 1.59) | <0.001d | 1.16 (1.08 to 1.24) | <0.001d | 1.13 (1.05 to 1.21) | 0.001d |
| Tiglylglycine | 1.45 (1.38 to 1.52) | <0.001d | 1.08 (1.01 to 1.14) | 0.02 | 1.06 (1.00 to 1.13) | 0.05 |
| Kynurenic acid | 1.73 (1.62 to 1.84) | <0.001d | 1.24 (1.13 to 1.35) | <0.001d | 1.21 (1.10 to 1.32) | <0.001d |
| Xanthosine | 1.35 (1.29 to 1.42) | <0.001d | 1.10 (1.05 to 1.16) | <0.001d | 1.13 (1.08 to 1.19) | <0.001d |
| Cinnamoylglycine | 1.26 (1.21 to 1.32) | <0.001d | 1.11 (1.06 to 1.17) | <0.001d | 1.11 (1.06 to 1.17) | <0.001d |
| Indoxyl sulfate | 1.69 (1.60 to 1.79) | <0.001d | 1.17 (1.08 to 1.26) | <0.001d | 1.15 (1.06 to 1.24) | <0.001d |
| p-Cresol sulfate | 1.34 (1.29 to 1.39) | <0.001d | 1.08 (1.02 to 1.15) | 0.01 | 1.08 (1.01 to 1.16) | 0.02 |
| Summary score | 1.97 (1.84 to 2.11) | <0.001d | 1.27 (1.15 to 1.39) | <0.001d | 1.28 (1.16 to 1.41) | <0.001d |
Total n=3207. CKD progression defined as 50% decline in eGFRCRIC from baseline, initiation of chronic dialysis, or kidney transplantation over follow-up. Results from the Cox proportional hazard model. HR, hazard ratio; CI, confidence interval; eGFRCRIC, glomerular filtration rate estimated based on serum creatinine and cystatin C concentrations, age, sex, and Black race using an equation that was developed in a subcohort of 1433 CRIC participants who underwent 125-Iothalamate GFR clearance studies.
Model 1 adjusted for age, race, sex, eGFRCRIC, and log-transformed 24-h urinary albumin excretion.
Model 2 additionally adjusted for clinical site, BMI, diabetes, smoking status, systolic BP, any cardiovascular disease, and angiotensin-converting enzyme inhibitors/angiotensin receptor blockers.
Hazard ratio expressed per 50% lower secretory-solute clearance or per 10-unit lower summary secretion score.
Statistically significant after correction for multiple comparisons using the Hommel method.
Figure 1.
Associations of kidney clearances of secretory solutes with CKD progression follow a curvilinear pattern (part A: pyridoxic acid; part B: kynurenic acid; part C: indoxyl sulfate). Hazard ratio adjusted for age, race, sex, eGFR, log-transformed 24-hour urinary albumin excretion, clinical site, BMI, diabetes, smoking status, systolic BP, any cardiovascular disease, and angiotensin-converting enzyme inhibitors/angiotensin receptor blockers. Hazard ratio predicted using restricted cubic splines with three knots placed at the first, second, and third quartiles of solute clearances. Vertical dashed lines represent the median of the secretory solutes clearances. CI, confidence interval.
Associations of Kidney Clearances of Secretory Solutes with Decline in eGFR
There were 3075 participants with at least one eGFR measurement after baseline (median, seven measurements; IQR, four to ten measurements; Supplemental Table 7). Lower kidney clearances of all secretory solutes were associated with a significantly faster eGFR decline after full adjustment and correction for multiple comparisons (Table 4). The largest effect size was observed for indoxyl sulfate. Each 50% lower clearance of this solute was associated with a 2% greater annual decline in eGFR.
Table 4.
Associations between kidney clearances of secretory solutes and slope of eGFR decline
| Solute | Base Modela | Model 1b | Model 2c | |||
|---|---|---|---|---|---|---|
| Percentage Difference (95% CI)d | P Value | Percentage Difference (95% CI)d | P Value | Percentage Difference (95% CI)d | P Value | |
| Hippurate | −0.72 (−0.95 to −0.50) | <0.001e | −0.73 (−0.96 to −0.49) | <0.001e | −0.70 (−0.94 to −0.47) | <0.001e |
| Pyridoxic acid | −2.01 (−2.30 to −1.71) | <0.001e | −2.04 (−2.35 to −1.72) | <0.001e | −2.03 (−2.34 to −1.71) | <0.001e |
| Dimethyluric acid | −0.61 (−0.83 to −0.39) | <0.001e | −0.63 (−0.87 to −0.40) | <0.001e | −0.62 (−0.85 to −0.39) | <0.001e |
| Trimethyluric acid | −0.63 (−0.83 to −0.43) | <0.001e | −0.65 (−0.86 to −0.44) | <0.001e | −0.63 (−0.84 to −0.42) | <0.001e |
| Isovalerylglycine | −1.52 (−1.84 to −1.20) | <0.001e | −1.55 (−1.88 to −1.21) | <0.001e | −1.54 (−1.87 to −1.20) | <0.001e |
| Tiglylglycine | −1.52 (−1.81 to −1.22) | <0.001e | −1.51 (−1.51 to −1.20) | <0.001e | −1.50 (−1.81 to −1.19) | <0.001e |
| Kynurenic acid | −2.03 (−2.39 to −1.67) | <0.001e | −2.04 (−2.42 to −1.65) | <0.001e | −2.02 (−2.40 to −1.63) | <0.001e |
| Xanthosine | −0.98 (−1.25 to −0.72) | <0.001e | −1.04 (−1.32 to −0.76) | <0.001e | −1.05 (−1.33 to −0.77) | <0.001e |
| Cinnamoylglycine | −0.84 (−1.07 to −0.61) | <0.001e | −0.84 (−1.08 to −0.59) | <0.001e | −0.83 (−1.07 to −0.58) | <0.001e |
| Indoxyl sulfate | −2.07 (−2.41 to −1.73) | <0.001e | −2.10 (−2.46 to −1.74) | <0.001e | −2.07 (−2.43 to −1.72) | <0.001e |
| p-Cresol sulfate | −1.44 (−1.72 to −1.17) | <0.001e | −1.38 (−1.67 to −1.08) | <0.001e | −1.37 (−1.66 to −1.07) | <0.001e |
| Summary score | −2.64 (−3.05 to −2.23) | <0.001e | −2.70 (−3.13 to −2.27) | <0.001e | −2.67 (−3.1 to −2.24) | <0.001e |
Total n=3075. Results from the linear mixed-effect model. CI, confidence interval; eGFRCRIC, glomerular filtration rate estimated based on serum creatinine and cystatin C concentrations, age, sex, and Black race using an equation that was developed in a subcohort of 1433 CRIC participants who underwent 125-Iothalamate GFR clearance studies.
Base model included log-transformed secretory-solute clearance, time in years, and interaction term between secretory clearance and time as independent variables and log-transformed eGFRCRIC during follow-up as the dependent variable.
Model 1 adjusted for baseline age, race, sex, log-transformed eGFRCRIC, and log-transformed 24-h urinary albumin excretion.
Model 2 additionally adjusted for baseline clinical site, BMI, diabetes, smoking status, systolic BP, any cardiovascular disease, and angiotensin-converting enzyme inhibitors/angiotensin receptor blockers.
Additional percentage annual change in eGFRCRIC per 50% lower baseline secretory solutes clearances or per 10-unit lower baseline summary secretion score.
Statistically significant after correction for multiple comparisons using the Hommel method.
Associations of Kidney Clearances of Secretory Solutes with Mortality
There were 1004 deaths over a median follow-up period of 9.6 years. Associations of kidney clearances of secretory solutes with mortality were attenuated by adjustment for eGFR, albuminuria, and demographics (Table 5). After full adjustment and accounting for multiple comparisons, lower kidney clearances of four solutes—isovalerylglycine, tiglylglycine, hippurate, and trimethyluric acid—were associated with a greater risk of death. Similar associations were observed for the composite outcome of CKD progression or all-cause death (Supplemental Table 8). When analyzed across categories of eGFR and albuminuria, mortality rates were higher for lower quartiles of the summary secretion score (Supplemental Table 9).
Table 5.
Associations between kidney clearances of secretory solutes and all-cause mortality
| Solute | Unadjusted | Model 1a | Model 2b | ||||
|---|---|---|---|---|---|---|---|
| HR (95% CI)c | P Value | HR (95% CI)c | P Value | HR (95% CI)c | P Value | ||
| Hippurate | 1.14 (1.10 to 1.18) | <0.001d | 1.04 (1.00 to 1.08) | 0.06 | 1.07 (1.02 to 1.11) | 0.002d | |
| Pyridoxic acid | 1.42 (1.35 to 1.50) | <0.001d | 1.12 (1.04 to 1.21) | 0.002d | 1.10 (1.02 to 1.19) | 0.01 | |
| Dimethyluric acid | 1.15 (1.11 to 1.20) | <0.001d | 1.03 (0.99 to 1.08) | 0.20 | 1.02 (0.97 to 1.06) | 0.47 | |
| Trimethyluric acid | 1.20 (1.15 to 1.24) | <0.001d | 1.07 (1.03 to 1.12) | 0.001d | 1.06 (1.02 to 1.11) | 0.004d | |
| Isovalerylglycine | 1.52 (1.44 to 1.62) | <0.001d | 1.25 (1.16 to 1.35) | <0.001d | 1.23 (1.14 to 1.32) | <0.001d | |
| Tiglylglycine | 1.45 (1.38 to 1.52) | <0.001d | 1.20 (1.12 to 1.28) | <0.001d | 1.19 (1.11 to 1.28) | <0.001d | |
| Kynurenic acid | 1.55 (1.45 to 1.67) | <0.001d | 1.12 (1.02 to 1.23) | 0.02 | 1.13 (1.03 to 1.24) | 0.01 | |
| Xanthosine | 1.26 (1.20 to 1.32) | <0.001d | 1.07 (1.01 to 1.13) | 0.02 | 1.06 (1.01 to 1.12) | 0.03 | |
| Cinnamoylglycine | 1.19 (1.14 to 1.24) | <0.001d | 1.05 (1.00 to 1.10) | 0.07 | 1.06 (1.01 to 1.12) | 0.02 | |
| Indoxyl sulfate | 1.42 (1.33 to 1.52) | <0.001d | 1.04 (0.96 to 1.13) | 0.36 | 1.06 (0.97 to 1.15) | 0.22 | |
| p-Cresol sulfate | 1.25 (1.19 to 1.32) | <0.001d | 0.98 (0.91 to 1.06) | 0.64 | 1.01 (0.93 to 1.08) | 0.87 | |
| Summary score | 1.75 (1.62 to 1.89) | <0.001d | 1.26 (1.13 to 1.40) | <0.001d | 1.27 (1.14 to 1.41) | <0.001d | |
Total n=3416. Results from the Cox proportional hazard model. HR, hazard ratio; CI, confidence interval; eGFRCRIC, glomerular filtration rate estimated based on serum creatinine and cystatin C concentrations, age, sex, and Black race using an equation that was developed in a subcohort of 1,433 CRIC participants who underwent 125-Iothalamate GFR clearance studies.
Model 1 adjusted for age, race, sex, eGFRCRIC, and log-transformed 24-h urinary albumin excretion.
Model 2 additionally adjusted for clinical site, BMI, diabetes, smoking status, systolic BP, any cardiovascular disease, and angiotensin-converting enzyme inhibitors/angiotensin receptor blockers.
Hazard ratio expressed per 50% lower secretory solute clearance or per 10-unit lower summary secretion score.
Statistically significant after correction for multiple comparisons using the Hommel method.
Sensitivity Analyses
Among the subset of 1187 CRIC study participants who completed iGFR clearance measurements at baseline, additional adjustment for iGFR only modestly affected the magnitude of associations between clearances of secretory solutes and CKD progression and all-cause mortality (Supplemental Tables 10 and 11). Additional adjustment for insulin, statins, loop diuretics, and thiazide diuretics had a minimal effect on the original associations in the full ancillary study cohort (Supplemental Table 12). Analysis of CKD progression defined by GFR estimated from the CKD Epidemiology Collaboration equation, instead of the CRIC study equation, yielded consistently larger effect sizes and lower P values (Supplemental Table 13). Plasma concentrations of secretory solutes alone were less strongly associated with CKD progression than their respective kidney clearances (Supplemental Table 14).
Discussion
In a national cohort study of CKD, we found lower kidney clearances of six candidate endogenous secretory solutes to be associated with greater risks of CKD progression after controlling for eGFR, albuminuria, and other established risk factors for kidney-function decline. Lower clearances of all solutes were also associated with faster eGFR decline in analyses of slope. Lower kidney clearances of four secretory solutes were associated with all-cause mortality. In sensitivity analyses, these associations were minimally attenuated by additional adjustment for directly measured GFR. Our findings support the hypothesis that the tubular clearance of secretory solutes provides additional information about kidney health beyond measurements of glomerular function alone.
Nine of the candidate secretory solutes evaluated in this study are substrates of OAT1 and OAT3, which belong to the solute carrier superfamily.21–23 OAT1 and OAT3 are secondary active transporters located on the basolateral membrane of proximal tubular cells that facilitate the influx of organic anions through exchange with α-ketoglutarate. The expression of OAT1/OAT3 is decreased in experimental models of CKD, possibly due to loss of proximal tubule cells and/or direct downregulation of OAT expression as an adaptive response to increased exposure to toxic solutes.22,24,25 On the apical surface, transporters from the ATP-binding cassette transporter superfamily, most notably multidrug resistance protein 4 and breast cancer resistance protein, harness the energy from ATP hydrolysis to extrude several of the secretory solutes evaluated in this study (e.g., indoxyl sulfate, p-cresol sulfate, and kynurenic acid) into the urine.26–29
Lower kidney clearances of secretory solutes may reflect relatively diminished renal blood flow through the peritubular capillaries, particularly for the most highly secreted solutes. Decreased renal blood flow is associated with kidney disease progression in autosomal dominant polycystic kidney disease and could reflect disease severity in other causes of CKD.30 Unfortunately, direct measurements of kidney blood flow using para-aminohippuric acid were not performed in the CRIC study and are cumbersome to obtain, promoting evaluation of the endogenous solutes assessed here. The active and coordinated processes required for proximal tubular secretion may also be uniquely susceptible to disease mechanisms that compromise the interstitial space, such as inflammation and fibrosis, or interfere with cellular energy generation by impairing mitochondrial function.31–34 Histologically, the severity of tubulointerstitial lesions, not glomerular injury, is most strongly associated with progression to ESKD.3 Contrasts between glomerular and tubular health are supported by the differences between secretory-solute clearances and eGFR observed in this study, and by heterogeneity in metabolic complications of CKD for a given level of GFR, such as hyperparathyroidism, anemia, and acidosis.4,5
Retained secretory solutes themselves could contribute to a decline in kidney function. Many protein-bound solutes are recognized uremic toxins that have been linked with CKD progression by inducing thrombosis, inflammation, vascular calcification, and endothelial dysfunction.35,36 Moreover, uremic toxins, such as indoxyl sulfate and p-cresol sulfate, are associated with activation of the renin-angiotensin-aldosterone system and kidney-tissue remodeling.37–39 Nonetheless, plasma concentrations of the selected secretory solutes alone, rather than their respective kidney clearances, were not significantly associated with CKD progression in this study.
These results confirm associations found in our previous single-site study with a smaller sample size, limited outcomes, and assessment of fewer secretory solutes.40 The tubular secretion of protein-bound solutes and drugs is an essential biologic kidney function that is plausibly disrupted by mechanisms of progressive kidney disease. Developing more expansive measurements of kidney functions that include secretory clearance could improve knowledge of the severity, complications, and prognosis of CKD. This study represents a first step in this process by demonstrating associations of the kidney clearances of suspected secretory solutes with the long-term progression of CKD. Follow-up studies are needed to determine whether estimates of tubular secretory clearance could improve clinical decision making, promote safer kidney drug-dosing strategies, or identify specific solutes that could be potential targets for future therapeutic interventions.
Strengths of our study include the evaluation of a broad group of patients with CKD who were recruited from multiple clinic sites; assessment of clinically relevant CKD progression outcomes over long-term follow-up; standardized measurements of study data, including 24-hour urine collections; and the use of a targeted LC-MS/MS procedure that was specifically developed for the secretory solutes of interest. Another strength is that the sensitivity analysis adjusting for direct measurements of GFR yielded largely similar results compared with our primary analyses. An important weakness of this study is estimation, rather than direct measurement, of tubular secretory clearances. There is no definitive method to prove that a substance is cleared exclusively by tubular secretion. Instead, secretory clearance was estimated by the total kidney clearances of specific solutes that are hypothesized to be cleared primarily by tubular secretion. Secretory clearance is supported by a high protein-binding percentage for some of the evaluated solutes: kynurenic acid (96%), cinnamoylglycine (95%), indoxyl sulfate (93%), p-cresol sulfate (96%), and pyridoxic acid (87%), implying minimal glomerular filtration. Many of these solutes are also poorly cleared with hemodialysis.41,42 Other solutes evaluated in this study demonstrated lower amounts of protein binding, but kidney clearances that were substantially higher than GFR, suggesting secretion as the major kidney mechanism of elimination. A second weakness is that the cause of kidney disease was self-reported in CRIC, motivating future studies that include kidney histology. The CRIC study excluded patients with a baseline eGFR of <20 ml/min per 1.73 m2, precluding investigation of the role of clearance of secretory solutes in patients with the most advanced stages of CKD.
Despite statistical significance, the observed associations between kidney clearances of secretory solutes and study outcomes were generally modest, limiting their use in prediction. eGFR was more strongly associated with CKD progression than clearances of secretory solutes in models that included both kidney functions. More robust associations of eGFR with CKD progression may be partly explained by the use of GFR itself to define this outcome, including the decision to initiate RRT to some degree. Decades of research have improved the precision and accuracy of GFR-estimating equations,43,44 whereas measurements of secretory-solute clearance in humans are emerging. The clearances evaluated in this study are subject to residual imprecision of the laboratory assays, diurnal variation in plasma concentrations, and miscollection of 24-hour urine samples, all of which may have diluted associations with outcomes.
In summary, we found lower kidney clearances of secretory solutes to be associated with CKD progression and all-cause mortality in a national cohort study of CKD. Kidney clearances of tubular secretory solutes may provide complementary information to existing measurements of GFR and albuminuria for the assessment of kidney health.
Disclosures
Dr. Becker reports grants from the National Institutes of Health (NIH), during the conduct of the study. Dr. Feldman reports grants from NIH, National Institute of Diabetes and Digestive and Kidney Diseases (NIH-NIDDK), during the conduct of the study; other from Kyowa Hakko Kirin Co., Ltd.; and other from American Journal of Kidney Disease, outside the submitted work. Dr. Hoofnagle reports grants from the NIH, during the conduct of the study; grants from Waters, Inc., outside the submitted work. Dr. Hsu reports grants from NIH-NIDDK, during the conduct of the study; and personal fees from EcoR1 Capital Fund, personal fees from Health Advances, personal fees from Ice Miller LLP, nonfinancial support from Microlife, personal fees and grants from Satellite Healthcare, and personal fees from UpToDate, outside the submitted work. Dr. Mehta reports other from Abbott Laboratories, other from AbbVie Inc., and other from Teva Pharmaceutical Industries, outside the submitted work. Dr. Lash reports grants from NIH, during the conduct of the study. Dr. Rahman reports grants from NIH, during the conduct of the study. Dr. Shafi reports personal fees from Hershey Medical Center, personal fees from Siemens, personal fees from University of California Irvine, personal fees from University of Mississippi Medical Center, and personal fees from University of Tennessee, outside the submitted work. Dr. Waikar reports grants and personal fees from Allena Pharmaceuticals, personal fees from Barron and Budd (versus Fresenius), personal fees from Bunch and James, personal fees from Cerus, personal fees from CVS, personal fees from GE Health Care, personal fees from GSK, personal fees from Harvard Clinical Research Institute (also known as Baim), personal fees from JNJ, personal fees from Kantum Pharma, personal fees from Mallinckrodt, personal fees from Mass Medical International, personal fees from Pfizer, personal fees from Public Health Advocacy Institute, personal fees from Roth Capital Partners, personal fees from Strataca, personal fees from Takeda, personal fees from Venbio, and personal fees from Wolters Kluewer, outside the submitted work. Dr. Zelnick reports grants from NIDDK, during the conduct of the study.
Funding
This work was supported by NIH grant R01 DK107931. Funding for the CRIC study was obtained under a cooperative agreement from the NIDDK (U01DK060990, U01DK060984, U01DK061022, U01DK061021, U01DK061028, U01DK060980, U01DK060963, and U01DK060902). In addition, this work was supported in part by the Perelman School of Medicine, University of Pennsylvania, Clinical and Translational Science Award NIH/National Center for Advancing Translational Sciences (NCATS) grant UL1TR000003; Johns Hopkins University grant UL1 TR-000424; University of Maryland General Clinical Research Center grant M01 RR-16500; Clinical and Translational Science Collaborative of Cleveland, School of Medicine, Case Western Reserve University grant UL1TR000439 from the NCATS component of the NIH and NIH Roadmap for Medical Research; Michigan Institute for Clinical and Health Research grant UL1TR000433; University of Illinois at Chicago grant CTSAUL1RR029879; Tulane University Centers of Biomedical Research Excellence (COBRE) award for Clinical and Translational Research in Cardiometabolic Diseases (P20 GM109036); and Kaiser Permanente NIH/National Center for Research Resources, Clinical and Translational Science Institute, University of California, San Francisco grant UL1 RR-024131.
Supplementary Material
Acknowledgments
The CRIC study investigators are Lawrence J. Appel (Johns Hopkins University), Dr. H.I. Feldman, Dr. A.S. Go, and Dr. Jiang He (Tulane University), Dr. James P. Lash (University of Illinois), Dr. Panduranga S. Rao (University of Michigan), Dr. Mahboob Rahman (Case Western Reserve University), and Dr. Raymond R. Townsend (University of Pennsylvania).
Dr. Kestenbaum, Dr. Feldman, and Dr. Lash designed the study; Dr. Chen, Dr. Wang, and Dr. Kestenbaum conducted literature search; Dr. Hoofnagle, Dr. Becker, and Dr. Kestenbaum collected data; Dr. Chen, Dr. Zelnick, Dr. Hoofnagle, Dr. Becker, and Dr. Kestenbaum analyzed data; all authors interpreted data; Dr. Chen and Dr. Kestenbaum drafted the manuscript; all authors revised and approved the final version of the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Contributor Information
Collaborators: Lawrence J. Appel, Harold I. Feldman, Alan S. Go, Jiang He, James P. Lash, Panduranga S. Rao, Mahboob Rahman, and Raymond R. Townsend
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2019080811/-/DCSupplemental.
Supplemental Table 1. Laboratory characteristics and variability of secretory solutes.
Supplemental Table 2. Kidney clearance and plasma concentration of secretory solutes by quartiles of the eGFR.
Supplemental Table 3. Correlations among the individual secretory solutes clearances.
Supplemental Table 4. Associations of baseline participant characteristics with the summary secretion score.
Supplemental Table 5. Secretory solute clearances by self-reported etiologies of CKD.
Supplemental Table 6. Adjusted incidence rates of CKD progression by quartiles of the summary secretion score and categories of baseline eGFR and 24-hour urinary albumin excretion.
Supplemental Table 7. Number of CRIC participants who completed follow-up visits for analyses of slope.
Supplemental Table 8. Associations between secretory solute clearances and the composite outcome of CKD progression and all-cause mortality.
Supplemental Table 9. Adjusted incidence rates of all-cause mortality by quartiles of the summary secretion score and categories of baseline eGFR and 24-hour urinary albumin excretion.
Supplemental Table 10. Associations between secretory solute clearances and CKD progression in the iGFR subcohort.
Supplemental Table 11. Associations between secretory solute clearances and all-cause mortality in the iGFR subcohort.
Supplemental Table 12. Associations between secretory solute clearances and CKD progression with additional adjustment for insulin, statins, and diuretics.
Supplemental Table 13. Associations between secretory solute clearances and CKD progression defined using eGFR from CKD-EPI equation.
Supplemental Table 14. Associations between secretory solute plasma concentration and CKD progression.
Supplemental Figure 1. Participant flow diagram.
Supplemental Figure 2. Distribution of the summary secretion score.
References
- 1.Centers for Disease Control and Prevention : National Chronic Kidney Disease Fact Sheet: General Information and National Estimates on Chronic Kidney Disease in the United States, 2010, Atlanta, GA, US Department of Health and Human Services, Centers for Disease Control and Prevention, 2010 [Google Scholar]
- 2.Kidney Disease: Improving Global Outcomes : KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 3: 1–150, 2013 [DOI] [PubMed] [Google Scholar]
- 3.Nath KA: Tubulointerstitial changes as a major determinant in the progression of renal damage. Am J Kidney Dis 20: 1–17, 1992. [DOI] [PubMed] [Google Scholar]
- 4.Levin A, Bakris GL, Molitch M, Smulders M, Tian J, Williams LA, et al.: Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: Results of the study to evaluate early kidney disease. Kidney Int 71: 31–38, 2007. [DOI] [PubMed] [Google Scholar]
- 5.Hsu CY, Propert K, Xie D, Hamm L, He J, Miller E, et al.; CRIC Investigators: Measured GFR does not outperform estimated GFR in predicting CKD-related complications. J Am Soc Nephrol 22: 1931–1937, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koepsell H, Endou H: The SLC22 drug transporter family. Pflugers Arch 447: 666–676, 2004. [DOI] [PubMed] [Google Scholar]
- 7.Nigam SK: What do drug transporters really do? Nat Rev Drug Discov 14: 29–44, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feldman HI, Appel LJ, Chertow GM, Cifelli D, Cizman B, Daugirdas J, et al.; Chronic Renal Insufficiency Cohort (CRIC) Study Investigators: The chronic renal insufficiency cohort (CRIC) study: Design and methods. J Am Soc Nephrol 14[Suppl 2]: S148–S153, 2003. [DOI] [PubMed] [Google Scholar]
- 9.Lash JP, Go AS, Appel LJ, He J, Ojo A, Rahman M, et al.; Chronic Renal Insufficiency Cohort (CRIC) Study Group: Chronic Renal Insufficiency Cohort (CRIC) study: Baseline characteristics and associations with kidney function. Clin J Am Soc Nephrol 4: 1302–1311, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bush KT, Wu W, Lun C, Nigam SK: The drug transporter OAT3 (SLC22A8) and endogenous metabolite communication via the gut-liver-kidney axis. J Biol Chem 292: 15789–15803, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rhee EP, Clish CB, Ghorbani A, Larson MG, Elmariah S, McCabe E, et al.: A combined epidemiologic and metabolomic approach improves CKD prediction. J Am Soc Nephrol 24: 1330–1338, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sirich TL, Aronov PA, Plummer NS, Hostetter TH, Meyer TW: Numerous protein-bound solutes are cleared by the kidney with high efficiency. Kidney Int 84: 585–590, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rivara MB, Zelnick LR, Hoofnagle AN, Newitt R, Tracy RP, Kratz M, et al.: Diurnal and long-term variation in plasma concentrations and renal clearances of circulating markers of kidney proximal tubular secretion. Clin Chem 63: 915–923, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anderson AH, Yang W, Hsu CY, Joffe MM, Leonard MB, Xie D, et al.; CRIC Study Investigators: Estimating GFR among participants in the chronic renal insufficiency cohort (CRIC) study. Am J Kidney Dis 60: 250–261, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hsu C, Propert K, Xie D, Hamm L, He J, Miller E, et al. : Measured GFR does not outperform estimated GFR in predicting CKD-related complications. J Am Soc Nephrol 22: 1931–1937, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cedillo-Couvert EA, Ricardo AC, Chen J, Cohan J, Fischer MJ, Krousel-Wood M, et al.; CRIC Study Investigators: Self-reported medication adherence and CKD progression. Kidney Int Rep 3: 645–651, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hsu JY, Roy JA, Xie D, Yang W, Shou H, Anderson AH, et al.; Chronic Renal Insufficiency Cohort (CRIC) Study Investigators: Statistical methods for cohort studies of CKD: Survival analysis in the setting of competing risks. Clin J Am Soc Nephrol 12: 1181–1189, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang W, Xie D, Anderson AH, Joffe MM, Greene T, Teal V, et al.; CRIC Study Investigators: Association of kidney disease outcomes with risk factors for CKD: Findings from the Chronic Renal Insufficiency Cohort (CRIC) study. Am J Kidney Dis 63: 236–243, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al.; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration): A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hommel G: A stagewise rejective multiple test procedure based on a modified Bonferroni test. Biometrika 75: 383–386, 1988 [Google Scholar]
- 21.Sugawara M, Mochizuki T, Takekuma Y, Miyazaki K: Structure-affinity relationship in the interactions of human organic anion transporter 1 with caffeine, theophylline, theobromine and their metabolites. Biochim Biophys Acta 1714: 85–92, 2005. [DOI] [PubMed] [Google Scholar]
- 22.Masereeuw R, Mutsaers HA, Toyohara T, Abe T, Jhawar S, Sweet DH, et al. : The kidney and uremic toxin removal: Glomerulus or tubule? Semin Nephrol, Vol. 34, 2014, pp 191–208 [DOI] [PubMed] [Google Scholar]
- 23.Nigam SK, Bush KT, Martovetsky G, Ahn S-Y, Liu HC, Richard E, et al.: The organic anion transporter (OAT) family: A systems biology perspective. Physiol Rev 95: 83–123, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Naud J, Michaud J, Beauchemin S, Hébert M-J, Roger M, Lefrancois S, et al.: Effects of chronic renal failure on kidney drug transporters and cytochrome P450 in rats. Drug Metab Dispos 39: 1363–1369, 2011. [DOI] [PubMed] [Google Scholar]
- 25.Torres AM, Mac Laughlin M, Muller A, Brandoni A, Anzai N, Endou H: Altered renal elimination of organic anions in rats with chronic renal failure. Biochimica et Biophysica Acta (BBA) 1740: 29–37, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Nigam SK, Wu W, Bush KT, Hoenig MP, Blantz RC, Bhatnagar V: Handling of drugs, metabolites, and uremic toxins by kidney proximal tubule drug transporters. Clin J Am Soc Nephrol 10: 2039–2049, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takada T, Yamamoto T, Matsuo H, Tan JK, Ooyama K, Sakiyama M, et al.: Identification of ABCG2 as an exporter of uremic toxin indoxyl sulfate in mice and as a crucial factor influencing CKD progression. Sci Rep 8: 11147, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mutsaers HA, Caetano-Pinto P, Seegers AE, Dankers AC, van den Broek PH, Wetzels JF, et al.: Proximal tubular efflux transporters involved in renal excretion of p-cresyl sulfate and p-cresyl glucuronide: Implications for chronic kidney disease pathophysiology. Toxicol In Vitro 29: 1868–1877, 2015. [DOI] [PubMed] [Google Scholar]
- 29.Dankers AC, Mutsaers HA, Dijkman HB, van den Heuvel LP, Hoenderop JG, Sweep FC, et al.: Hyperuricemia influences tryptophan metabolism via inhibition of multidrug resistance protein 4 (MRP4) and breast cancer resistance protein (BCRP). Biochim Biophys Acta 1832: 1715–1722, 2013. [DOI] [PubMed] [Google Scholar]
- 30.King BF, Torres VE, Brummer ME, Chapman AB, Bae KT, Glockner JF, et al.; Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease (CRISP): Magnetic resonance measurements of renal blood flow as a marker of disease severity in autosomal-dominant polycystic kidney disease. Kidney Int 64: 2214–2221, 2003. [DOI] [PubMed] [Google Scholar]
- 31.Stuveling EM, Hillege HL, Bakker SJ, Gans RO, De Jong PE, De Zeeuw D: C-reactive protein is associated with renal function abnormalities in a non-diabetic population. Kidney Int 63: 654–661, 2003. [DOI] [PubMed] [Google Scholar]
- 32.Rockey DC, Bell PD, Hill JA: Fibrosis--a common pathway to organ injury and failure. N Engl J Med 372: 1138–1149, 2015. [DOI] [PubMed] [Google Scholar]
- 33.Ross R: Atherosclerosis--an inflammatory disease. N Engl J Med 340: 115–126, 1999. [DOI] [PubMed] [Google Scholar]
- 34.Hall AM, Unwin RJ: The not so ‘mighty chondrion’: Emergence of renal diseases due to mitochondrial dysfunction. Nephron, Physiol 105: 1–10, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Chen WT, Chen YC, Hsieh MH, Huang SY, Kao YH, Chen YA, et al.: The uremic toxin indoxyl sulfate increases pulmonary vein and atrial arrhythmogenesis. J Cardiovasc Electrophysiol 26: 203–210, 2015. [DOI] [PubMed] [Google Scholar]
- 36.Duranton F, Cohen G, De Smet R, Rodriguez M, Jankowski J, Vanholder R, et al.; European Uremic Toxin Work Group: Normal and pathologic concentrations of uremic toxins. J Am Soc Nephrol 23: 1258–1270, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saito S, Yisireyili M, Shimizu H, Ng HY, Niwa T: Indoxyl sulfate upregulates prorenin expression via nuclear factor-κB p65, signal transducer and activator of transcription 3, and reactive oxygen species in proximal tubular cells. J Ren Nutr 25: 145–148, 2015. [DOI] [PubMed] [Google Scholar]
- 38.Sanaka T, Akizawa T, Koide K, Koshikawa S: Protective effect of an oral adsorbent on renal function in chronic renal failure: Determinants of its efficacy in diabetic nephropathy. Ther Apher Dial 8: 232–240, 2004. [DOI] [PubMed] [Google Scholar]
- 39.Sun CY, Hsu HH, Wu MS: p-Cresol sulfate and indoxyl sulfate induce similar cellular inflammatory gene expressions in cultured proximal renal tubular cells. Nephrol Dial Transplant 28: 70–78, 2013. [DOI] [PubMed] [Google Scholar]
- 40.Suchy-Dicey AM, Laha T, Hoofnagle A, Newitt R, Sirich TL, Meyer TW, et al.: Tubular secretion in CKD. J Am Soc Nephrol 27: 2148–2155, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kalim S, Wald R, Yan AT, Goldstein MB, Kiaii M, Xu D, et al.: Extended duration nocturnal hemodialysis and changes in plasma metabolite profiles. Clin J Am Soc Nephrol 13: 436–444, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sirich TL, Fong K, Larive B, Beck GJ, Chertow GM, Levin NW, et al.; Frequent Hemodialysis Network (FHN) Trial Group: Limited reduction in uremic solute concentrations with increased dialysis frequency and time in the Frequent Hemodialysis Network Daily Trial. Kidney Int 91: 1186–1192, 2017. [DOI] [PubMed] [Google Scholar]
- 43.Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, et al.; CKD-EPI Investigators: Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 367: 20–29, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D; Modification of Diet in Renal Disease Study Group : A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Ann Intern Med 130: 461–470, 1999. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.