Figure 1.
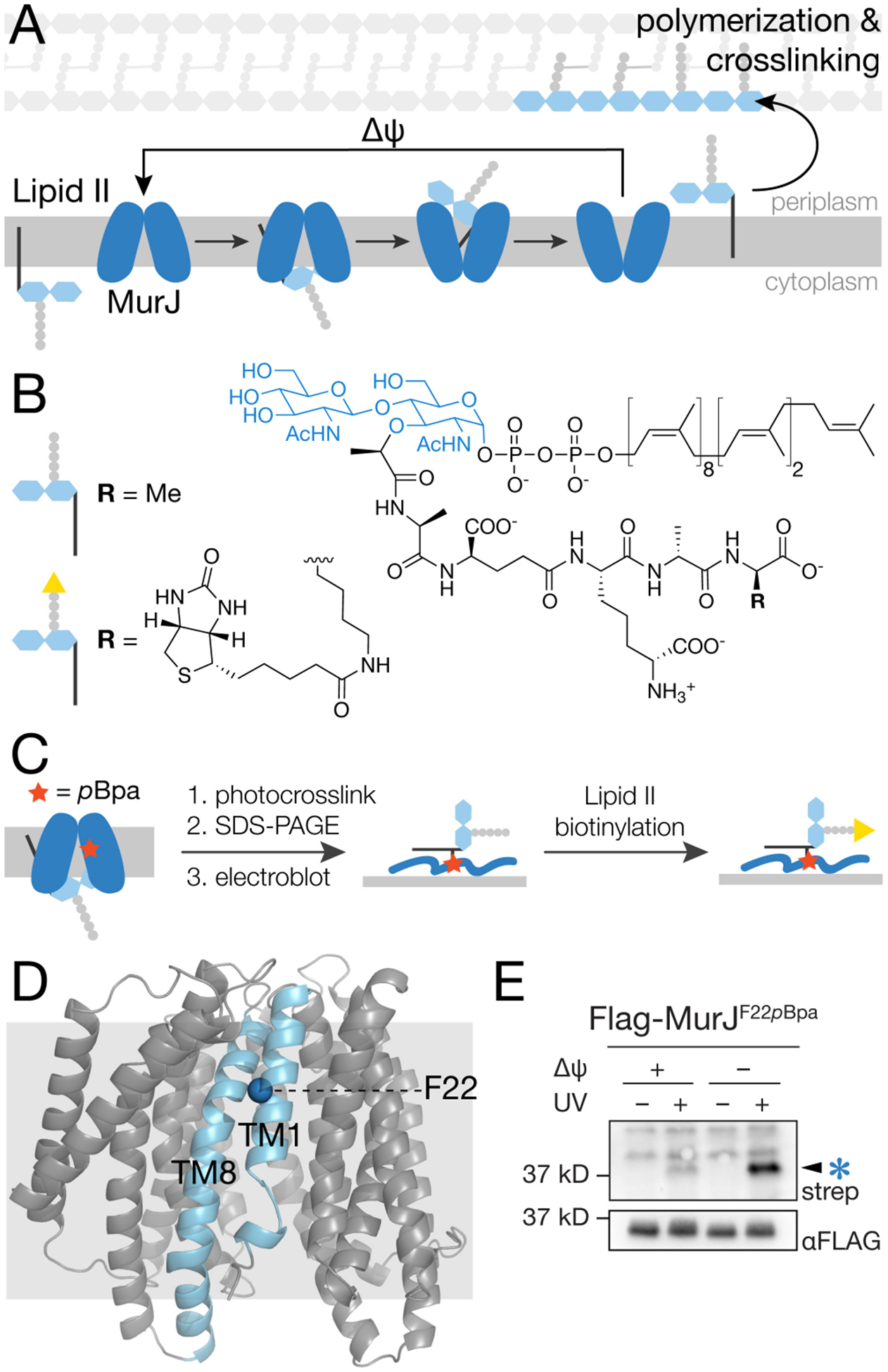
Photocrosslinking of MurJF22pBpa in cells produces an adduct that is detected using a method that specifically labels Lipid II (a) Lipid II is transported from the cytoplasm to the periplasm by MurJ via conformational changes driven by membrane potential (Δψ). (b) Structure of E. coli Lipid II and its biotinylated derivative. (c) Schematic showing the method to detect MurJ-Lipid II adducts. MurJ containing the unnatural amino acid pBpa (red star) is UV-irradiated to photocrosslink bound Lipid II. After SDS-PAGE and electroblotting, the Lipid II crosslinked to MurJ is biotinylated by D-amino acid exchange using PBP4, enabling detection with streptavidin-HRP.17 (d) Crystal structure of E. coli MurJ in the inward-facing conformation (PDB 6CC4)10 showing location of F22 in transmembrane helix 1 (TM1). (e) A UV-dependent band (arrow) was detected in a streptavidin-HRP blot after irradiating cells expressing MurJF22pBpa and labeling to detect Lipid II as in (c). Intensity of this band increased substantially when Lipid II was pre-accumulated on the inner leaflet of the cytoplasmic membrane (compare lanes 2 and 4). To accomplish this, cells were treated with 100 μM CCCP for 5 min and then diluted 100-fold to restore membrane potential just prior to irradiation. See also Figures S1–S3.
