Figure 3. Guanine Nucleotide-free Gα Stabilized by Ric-8A Adopts an Open Conformation.
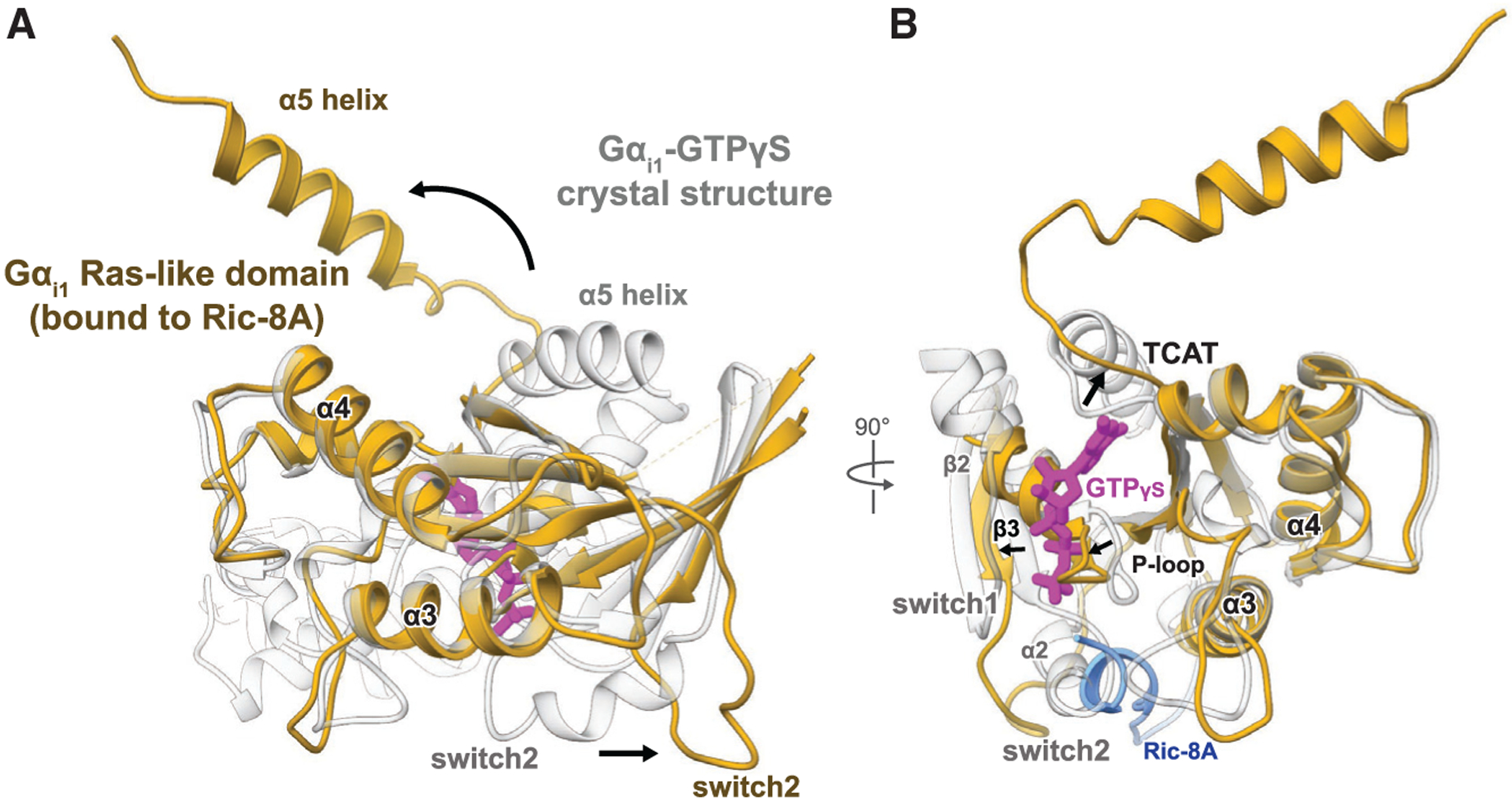
Comparison of the Gαi1 structures bound to the GTP analog 5′-guanosine-diphosphate-monothiophosphate (GTPgS) (PDB: 1GIA) and to Ric-8A. Gαi1 adopts a distinct conformation when bound to Ric-8A (gold) from that of the canonical GTP bound state (white).
(A) The α5 helix in the Ric-8A-Gαi1 complex is rotated more than 90 away from its position in the Gαi1-GTPγS crystal structure. The Gα switch2 motif is displaced outward compared to its conformation in the Ga-GTPγS structure. The helical segment of Ric-8A interacting with switch2 and the a3 helix of Gα is colored blue, and the rest of Ric-8A was omitted for clarity.
(B) The β6-a5 loop containing the TCAT motif is displaced 3–5Å outward from its position for guanine nucleotide coordination (thick arrow), and the interface between the α-helical and Ras-like domain is disordered together with the a2 helix and b2 strand.
