Figure 4. Ric-8A Phosphorylated Residues S435 and T440 Stabilize the R8-R9 ARM/HEAT Interaction with the Ras-like Domain Core.
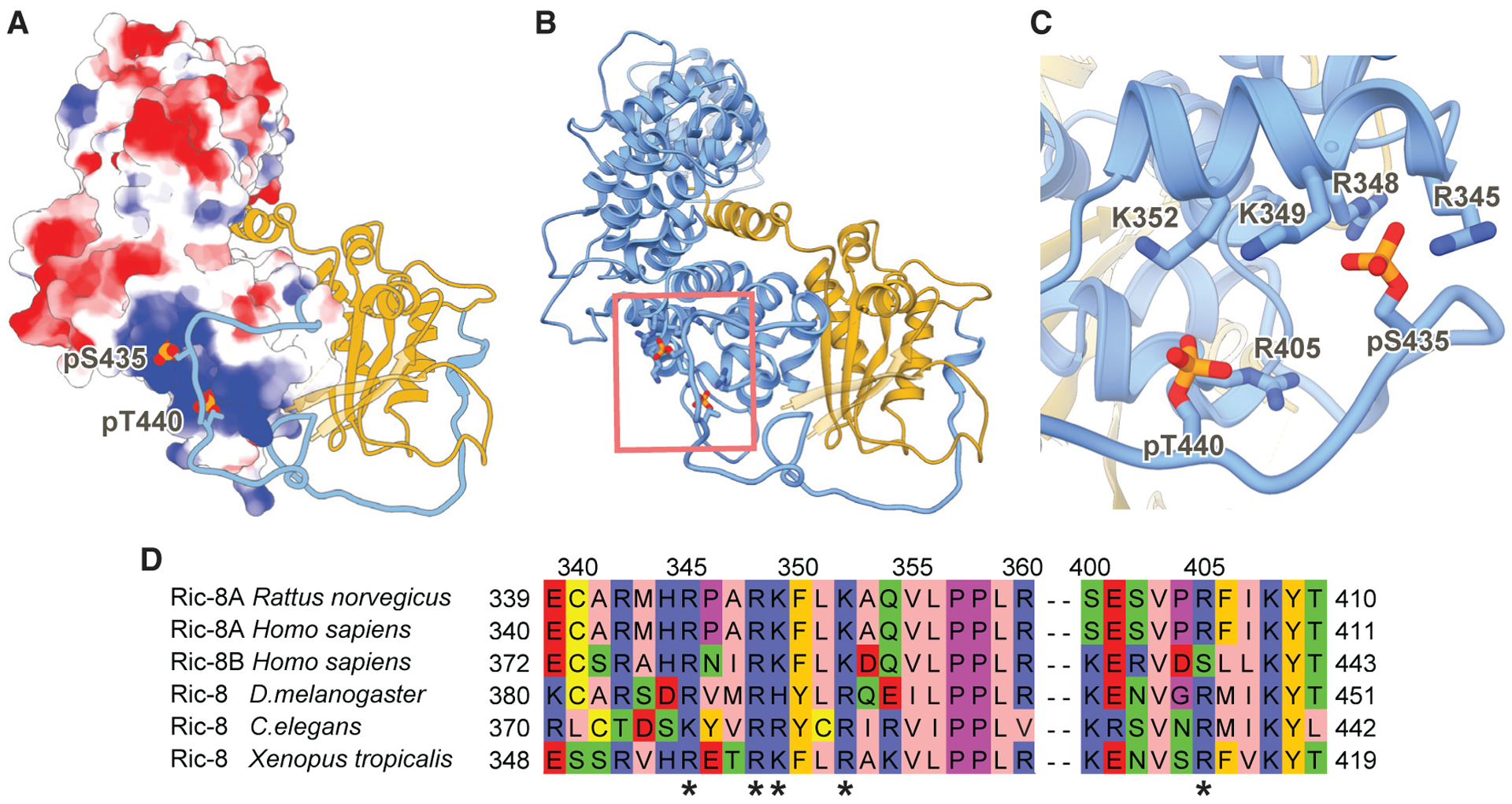
(A) Electrostatic surface representation of Ric-8A 1–421 (8 to +8 kT/e electrostatic potential is colored from red to blue respectively). Ric-8A residues 422–482 and Gαi1 are shown as ribbon models in blue and gold, respectively. Phosphorylated residues S435 and T440 are shown as spheres.
(B) Ribbon diagram of Ric-8A 1–482 (blue) and Gαi1 (gold) of the phosphorylated, full-length Ric-8A-Gαi1 complex. Phosphorylated residues S435 and T440 are shown as sticks.
(C) Phosphorylated residues S435 and T440 bind to conserved positively charged residues of Ric-8A.
(D) Sequence alignment of Ric-8 residues interacting with the phosphorylated S435 and T440 sites. The positively charged interacting residues (*) are highly conserved in both mammalian Ric-8 isoforms and across species.
