Figure 7. Proposed Mechanism for Ric-8A Chaperoning of Gα.
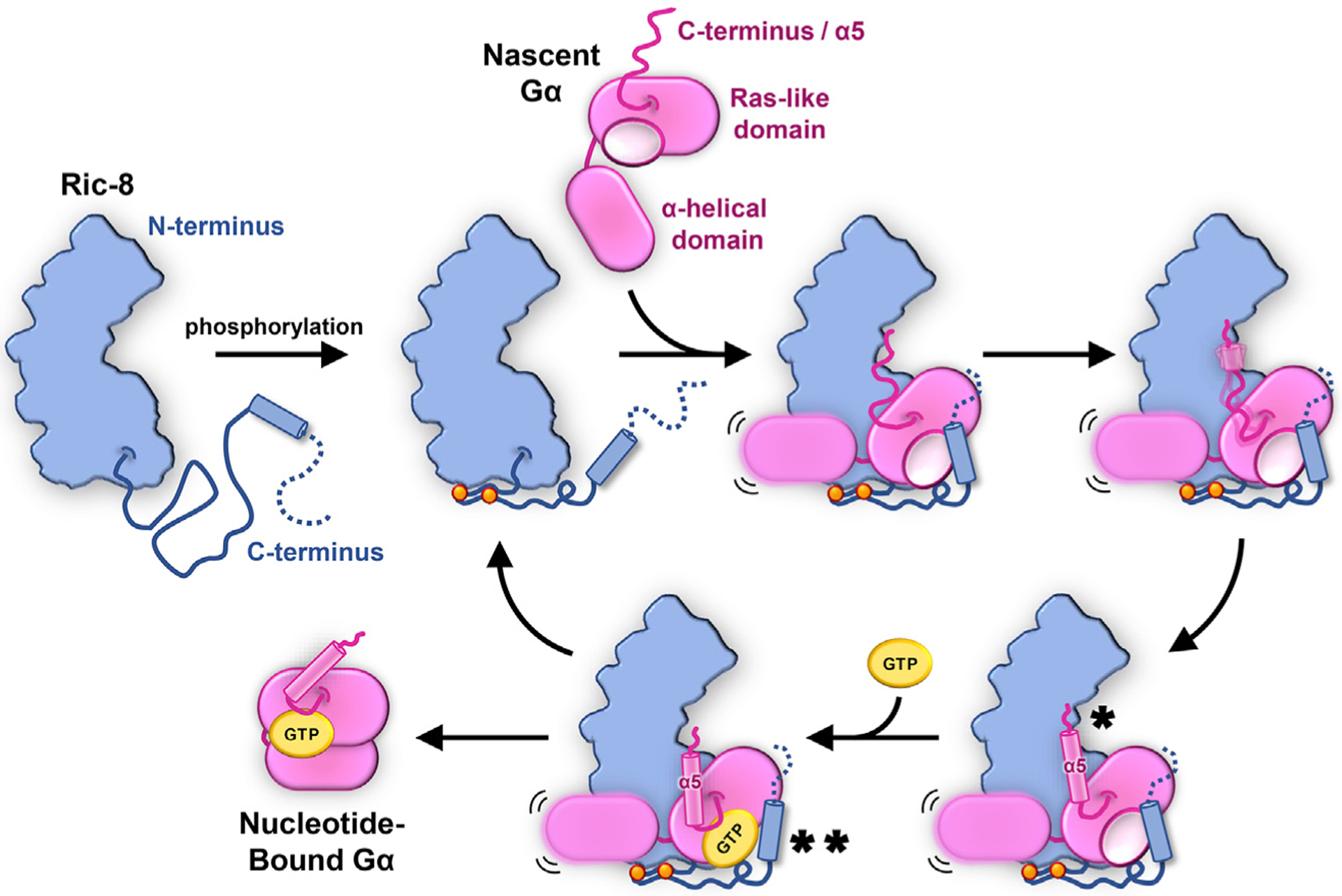
Dual phosphorylation of Ric-8 within a conserved acidic region (orange spheres) facilitates an intramolecular interaction that results in optimal positioning for Gα Ras-like domain engagement by Ric-8. Nascent, nucleotide-free Gα binds Ric-8 either co-translationally or shortly following release from the ribosome. Key interactions occur between the concave surface of the Ric-8 core and the a5-helix of Gα (*) and between a C-terminal helix of Ric-8 with Gα switch2 (**). The recognition of the final C-terminal residue of Gα by Ric-8 serves as a prerequisite for a5-helix folding (*) and positioning of the b6-a5 loop (TCAT motif) to engage GTP (**), thereby releasing properly folded Gα and freeing Ric-8 for subsequent rounds of chaperoning.
