Figure 1.
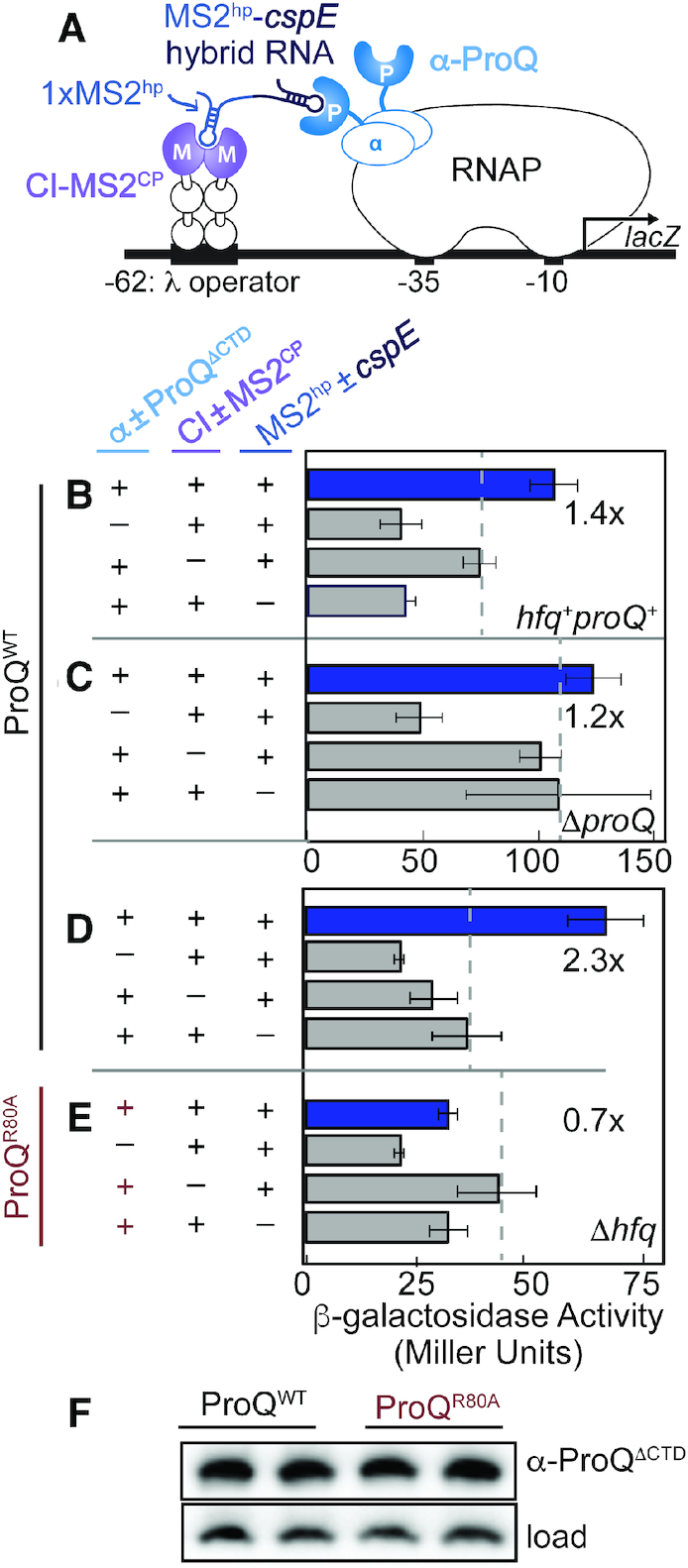
Adaptation of an E. coli bacterial three-hybrid (B3H) interaction to detect ProQ-RNA interactions. (A) Design of B3H system to detect interaction between ProQ and an RNA (cspE 3′UTR). Interaction between protein moiety ProQ and RNA moiety cspE fused, respectively, to the NTD of the alpha subunit of RNAP (α) and to one copy of the MS2 RNA hairpin (MS2hp) activates transcription from test promoter, which directs transcription of a lacZ reporter gene. The test promoter (plac-OL2-62), which bears the λ operator OL2 centered at position –62 relative to the transcription start site, is present on a single copy F’ episome. The RNA-binding moiety MS2CP is fused to λCI (CI-MS2CP) to tether the hybrid RNA (MS2hp-cspE) to the test promoter. Compatible plasmids direct the synthesis of the α-fusion protein (under the control of an IPTG-inducible promoter), the CI-MS2CP adapter protein (under the control of a constitutive promoter; pCW17) and the hybrid RNA (under the control of an arabinose-inducible promoter). (B–E) Results of β-galactosidase assays performed with wild type (B; hfq+proQ+), ΔproQ (C) or Δhfq (D,E) reporter strain cells containing three compatible plasmids: one (α±ProQΔCTD that encoded α alone (−) or the α-ProQΔCTD (+) fusion protein (resi = 2–176, WT or an R80A mutant), another (CI±MS2CP) that encoded λCI alone (−) or the λCI-MS2CP fusion protein (+), and a third (MS2hp±cspE) that encoded a hybrid RNA with the 3′ UTR of cspE (pSP10, final 85 nts) following one copy of an MS2hp moiety (+) or an RNA that contained only the MS2hp moiety (−). Cells were grown in the presence of 0.2% arabinose and 50 μM IPTG (see Methods). All subsequent assays were performed in Δhfq reporter strain cells. Bar graphs show the averages of three independent measurements and standard deviations. (F) Samples from (D) and (E) were analyzed by Western blot and probed with an anti-ProQ antibody detect α-ProQΔCTD fusion protein (α-ProQ). A cross-reacting band independent of the presence of endogenous ProQ or α-ProQ fusion protein is used as a loading control (load; see Supplementary Figure S3). Duplicate biological samples are shown.
