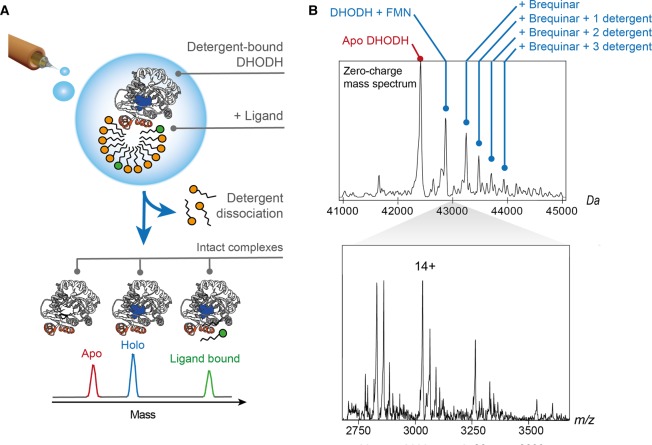
Figure 2. nMS of the peripheral membrane protein DHODH shows concomitant binding of cofactor, inhibitor, and detergent molecules.
(A) The nMS approach used for detergent-solubilized integral membrane proteins facilitates the analysis of intact DHODH complexes. Release of the desolvated protein from detergent micelles by collisional activation preserves interactions with the FMN cofactor as well as exogenous ligands. (B) nESI-MS of DHODH in the presence of detergent and Brequinar shows a series of peaks corresponding to the protein with its FMN cofactor in complex with one Brequinar molecule and zero, one, two, or three detergent molecules. The deconvoluted spectrum is shown above the corresponding mass spectrum. Adapted from reference [44] with permission. Copyright 2018 Elsevier.