Abstract
Type 2 diabetic nephropathy (T2DN) progresses with an increasingly inflammatory milieu, wherein various immune cells are relevant. Herein, we investigated the levels of myeloid-derived suppressor cells (MDSCs) and their clinical implication in patients with T2DN. A total of 91 subjects (T2DN, n=80; healthy, n=11) were recruited and their PBMCs were used for flow cytometric analysis of polymorphonuclear (PMN-) and monocytic (M-) MDSCs, in addition to other immune cell subsets. The risk of renal progression was evaluated according to the quartiles of MDSC levels using the Cox model. The proportion of MDSCs in T2DN patients was higher than in healthy individuals (median, 6.7% vs. 2.5%). PMN-MDSCs accounted for 96% of MDSCs, and 78% of PMN-MDSCs expressed Lox-1. The expansion of PMN-MDSCs was not related to the stage of T2DN or other kidney disease parameters such as glomerular filtration rate and proteinuria. The production of ROS in PMN-MDSCs of patients was higher than in neutrophils of patients or in immune cells of healthy individuals, and this production was augmented under hyperglycemic conditions. The 4th quartile group of PMN-MDSCs had a higher risk of renal progression than the 1st quartile group, irrespective of adjusting for multiple clinical and laboratory variables. In conclusion, PMN-MDSCs are expanded in patients with T2DN, and may represent as an immunological biomarker of renal progression.
Keywords: Diabetic nephropathy, End-stage renal disease, Immunology, Monocyte, Myeloid-derived suppressor cell
INTRODUCTION
Diabetic nephropathy (DN) is an important health condition because it is the leading cause of end-stage renal disease worldwide (1). The proportion of patients with DN has been increasing and reached up to 50% of patients with end-stage renal disease (2), with frequently encountering risks of higher morbidity and mortality (3,4). When these burdens in addition to socioeconomic illness are considered (5), development of an appropriate therapeutic approach for DN is a critical issue, yet there are no reversing or targeting agents except for supportive care such as glycemic control and anti-hypertensive agents.
The disease progression of DN can be classified by decreased kidney function (graded 1–5) and proteinuria (graded A1–3) (6). These progressions are clinically heterogeneous and may have different courses depending on patient status (7). This feature may be because the mechanism of DN is heterogeneous and not determined, and includes metabolic, hemodynamic, and oxidative stress processes, which interact with one another (8). The inflammatory milieu is related to the progression of DN, and several immune cell subsets play roles in DN, contributing to dysregulated metabolism, cell stress, and chronic inflammation (9). Tracking immune cell subsets may be helpful because specific subsets may underlie the progression of DN and thus represent potential novel targets to abrogate the disease.
Myeloid-derived suppressor cells (MDSCs) are a regulatory immune cell subset with the ability to suppress other immune cells including T, B, and NK cells (10). MDSCs are expanded in the tumor environment and can promote the severity and metastasis of tumors (11). There are 2 representative subtypes of MDSCs: polymorphonuclear MDSCs (PMN-MDSCs) and monocytic MDSC (M-MDSCs). Intriguingly, their detection is not limited to patients with tumors and they are frequently observed in several pathologic conditions such as autoimmune diseases (12,13). Patients with type 1 diabetes mellitus display an increased number of MDSCs in peripheral blood (14), but this cell type has not been comprehensively investigated in type 2 DN (T2DN). Herein, we investigated the expansion of the MDSC subset and its relationship with progression in a cohort of patients with T2DN.
MATERIALS AND METHODS
Study subjects
The study protocol complied with the ethical principles of the Declaration of Helsinki and received full approval from the Institutional Review Boards of Seoul National University Hospital (H-1702-049-831). A total of 80 patients who had been clinically diagnosed with T2DN were recruited from July 2017 to February 2018. To compare the proportion of immune subsets, 11 healthy individuals without evidence of kidney disease, hypertension, or diabetes mellitus were recruited during the same period. All study subjects provided written informed consent for the donation and use of their specimens in the present study.
Study variables and outcome
Clinical data on age, sex, and medications such as metformin, sulfonylurea, meglitinide, thiazolidinedione, dipeptidyl peptidase-4 inhibitor, glucagon-like peptide-1 receptor agonist, and insulin were collected. Laboratory findings, including serum creatinine, glucose, hemoglobin A1c, and random urine protein-to-creatinine ratio (uPCR) were obtained. The estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation (15). Renal progression as a primary outcome was defined when a doubling of serum creatinine, ≥50% decrease of eGFR, or end-stage renal disease (i.e., dialysis or kidney transplantation) occurred during the follow-up period.
Analysis of immune cells in peripheral blood samples
PBMCs were immediately isolated from heparinized whole blood by density gradient centrifugation with Ficoll-Paque (GE Healthcare, Chicago, IL, USA). Cells were washed, resuspended in staining buffer consisting of 2% horse serum and 0.05% sodium azide, blocked with anti-human Fc receptor binding inhibitor (eBioscience, San Diego, CA, USA), and then incubated with primary Abs. Samples were processed using a BD Fortessa™ machine (BD Biosciences, San Jose, CA, USA) and analyzed with FlowJo software (FlowJo, Ashland, OR, USA). Singlet cells were selected based on the scatter profiles, and dead cells were stained with DAPI (Molecular Probes, Eugene, OR, USA) and excluded from the analysis. The Abs used for flow cytometry are listed in Supplementary Table 1.
ROS production
Production of ROS in PBMCs and neutrophils from T2DN patients (n=18) and healthy individuals (n=8) were measured using the oxidation-sensitive dye, DCFDA (Invitrogen, Carlsbad, CA, USA). In brief, cells were labeled for surface markers, washed, and incubated at 37°C in serum-free RPMI media with 3 μM of DCFDA for 30 min. ROS production was measured by flow cytometry and presented as mean fluorescence intensity. In another experiment, PMN-MDSCs from patients with T2DN were cultured with indicated glucose concentrations for 12 h and their ROS production was measured as stated above.
Statistical analysis
All analyses and calculations were performed using SPSS (version 23.0; IBM Corp., Armonk, NY, USA) and GraphPad Prism (version 7.0; GraphPad Software, Inc., La Jolla, CA, USA). Categorical and continuous variables are expressed as proportions and the means±SD for normally distributed variables and as the median with interquartile range for non-normally distributed variables. The normality of distribution was analyzed via the Kolmogorov-Smirnov test. The χ2 test was used to compare categorical variables (Fisher's exact test, if not applicable). The Student's t-test or the Mann-Whitney U test was used to compare continuous variables with or without normal distributions, respectively. The correlation coefficient between continuous variables was measured using Pearson's correlation test. Kaplan-Meier survival curves were constructed and compared using the log-rank test. A Cox proportional hazards regression model was applied to calculate hazard ratios of renal progression. All p-values were 2-sided, and values <0.05 were considered significant.
RESULTS
Baseline characteristics
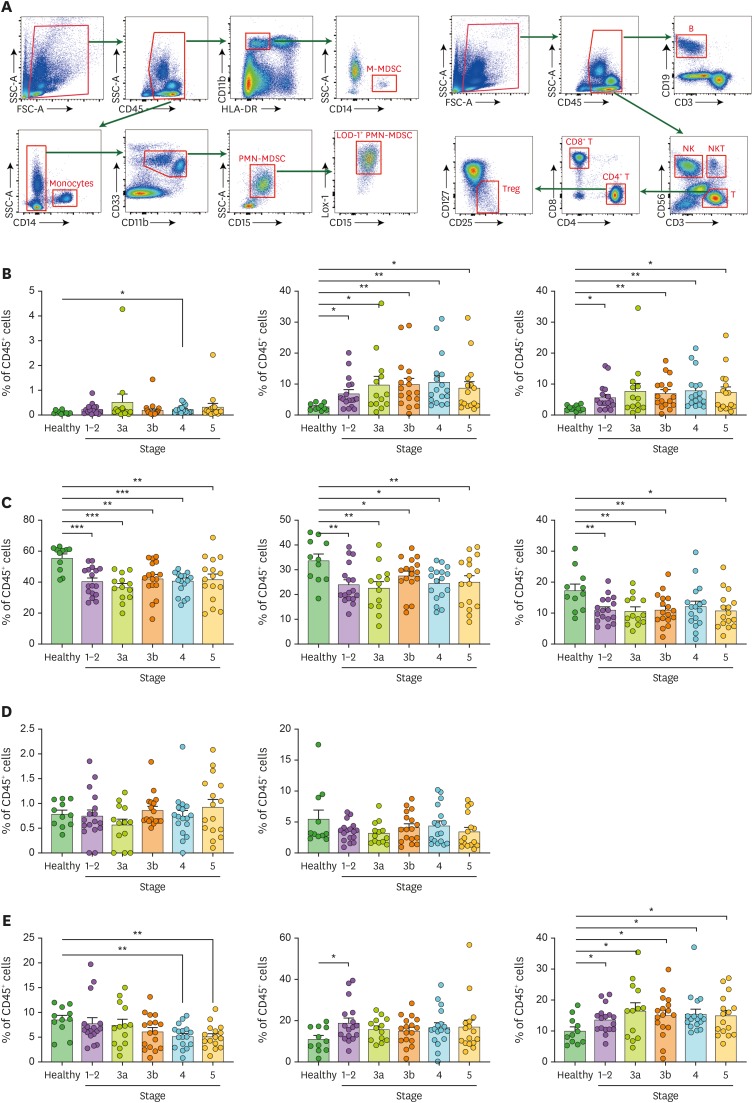
Table 1 shows baseline characteristics of patients with T2DN. The mean age was 69±9 years, and 63.7% were male. The median value of eGFR was 36.9 ml/min/1.73 m2 (17.1–54.2 ml/min/1.73 m2). When peripheral blood immune subsets were analyzed (Table 1 and Supplementary Table 2), the main subsets were CD3+ T cells, NK cells, and monocytes. Most MDSCs belonged to the PMN subset, whereas the proportion of M-MDSC was less than 1% of immune cells. The 78% of PMN-MDSC additionally expressed Lox-1 (i.e., Lox-1+ PMN-MDSCs).
Table 1. Baseline characteristics and immune profiling according to the progression of diabetic nephropathy.
| Variables | Total (n=80) | Non-progression (n=60) | Progression (n=20) | p | |
|---|---|---|---|---|---|
| Age (years) | 69.0±8.8 | 69.8±8.5 | 66.5±9.1 | 0.159 | |
| Male (%) | 63.7 | 63.3 | 65.0 | 0.893 | |
| Weight (kg) | 68.1±11.7 | 69.9±11.8 | 62.6±9.7 | 0.002 | |
| Comorbidities (%) | |||||
| History of ischemic heart disease | 23.8 | 21.7 | 30.0 | 0.448 | |
| History of stroke | 8.8 | 8.3 | 10.0 | 0.819 | |
| Diabetic retinopathy | 32.5 | 31.7 | 35.0 | 0.783 | |
| Laboratory findings | |||||
| Creatinine (mg/dl) | 1.7 (1.2–2.9) | 1.5 (1.1–2.2) | 3.6 (2.7–5.4) | <0.001 | |
| eGFR (ml/min/1.73 m2) | 36.9 (17.1–54.2) | 43.3 (29.8–61.4) | 13.7 (9.8–21.8) | <0.001 | |
| Random uPCR (g/g) | 1.0 (0.2–4.1) | 0.5 (0.1–1.8) | 5.7 (2.1–9.0) | <0.001 | |
| Fasting glucose (mg/dL) | 136.3±41.1 | 141.6±43.4 | 120.2±28.4 | 0.015 | |
| Hemoglobin A1c (%) | 7.0±1.2 | 7.2±1.2 | 6.5±1.1 | 0.033 | |
| Medications (%) | |||||
| Metformin | 50.0 | 55.0 | 35.0 | 0.121 | |
| Sulfonylurea | 36.3 | 33.3 | 45.0 | 0.347 | |
| Meglitinide | 2.5 | 3.3 | 0 | 0.408 | |
| Thiazolidinedione | 2.5 | 3.3 | 0 | 0.408 | |
| DPP4 inhibitor | 56.3 | 53.3 | 65.0 | 0.362 | |
| GLP1 receptor agonist | 0 | 0 | 0 | NA | |
| Insulin | 31.3 | 31.7 | 30.0 | 0.889 | |
| Immune cell subset (%) | |||||
| CD3+ T cells | 40.5±10.7 | 40.5±10.7 | 40.7±11.0 | 0.943 | |
| CD3+ CD4+ T cells | 25.1±8.4 | 25.4±8.3 | 24.5±8.7 | 0.679 | |
| CD3+ CD8+ T cells | 11.1±5.4 | 10.9±5.2 | 11.7±5.9 | 0.603 | |
| CD3+ CD4+ CD25+ CD127low/− Treg cells | 0.8±0.5 | 0.8±0.5 | 0.7±0.5 | 0.691 | |
| CD3+ CD56+ NKT cells | 3.4 (1.8–5.1) | 3.4 (1.8–5.3) | 3.2 (1.4–4.4) | 0.420 | |
| CD19+ B cells | 6.3±3.6 | 6.8±3.9 | 4.7±1.7 | 0.022 | |
| CD56+ NK cells | 16.7±9.2 | 17.7±9.6 | 13.8±7.5 | 0.068 | |
| CD14+ monocytes | 15.0±6.6 | 14.7±6.8 | 15.9±6.0 | 0.463 | |
| M-MDSCs | 0.2 (0.1–0.3) | 0.2 (0.2–0.3) | 0.2 (0.1–0.3) | 0.356 | |
| PMN-MDSCs | 6.5 (3.7–11.9) | 5.6 (3.5–10.5) | 9.7 (4.4–22.5) | 0.016 | |
| Lox-1+ PMN-MDSCs | 5.1 (3.0–9.6) | 4.5 (2.7–8.5) | 8.8 (3.9–16.7) | 0.004 | |
DPP4, dipeptidyl peptidase-4; GLP1, glucagon-like peptide-1.
Comparison of immune cell subsets between patients and healthy individuals
Gating strategies for immune cell subsets are shown in Fig. 1A. While the proportion of M-MDSCs did not differ between T2DN patients and healthy individuals except for those with stage 4 T2DN, the proportion of PMN-MDSC was significantly higher in patients with T2DN than in healthy individuals (Fig. 1B). Lox-1, which has been known to distinguish the population of human PMN-MDSCs from neutrophils in cancer patients, was further analyzed (16,17). The proportion of Lox-1+ PMN-MDSCs was elevated in patients with T2DN compared with healthy individuals, except for stage 3a. The overall trend in the absolute cell number results was similar to that in the proportion results, which is shown in Supplementary Fig. 1.
Figure 1. Profiling of immune cell subsets of patients with T2DN. (A) Gating strategies for peripheral blood immune cells. Proportions of (B) M-, PMN-, and Lox-1+ PMN-MDSCs, (C) total, CD4+, and CD8+ T cells, (D) regulatory and NK T cells, (E) B and NK cells, and monocytes in patients and healthy individuals.
*p<0.05; **p<0.01; ***p<0.001.
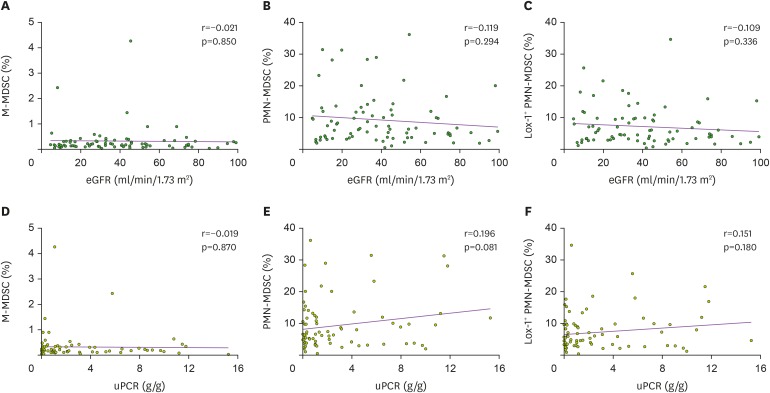
When other immune cell subsets were evaluated, the proportions of total, CD4+, and CD8+ T cells decreased in T2DN patients compared with healthy individuals (Fig. 1C). Other T cell subsets such as Treg and NKT cells did not differ between patients and healthy individuals (Fig. 1D). The proportion of B cells decreased in patients with advanced stages of T2DN, whereas that of NK cells increased in patients with early stage T2DN compared with healthy individuals (Fig. 1E). The calculated proportions of immune cell subsets are shown in Supplementary Table 2. When the relationships between MDSC and the parameters of kidney dysfunctions were explored, none of the MDSC subtypes had a linear relationship with the levels of eGFR (Fig. 2A-C) and uPCR (Fig. 2D-F). Collectively, PMN-MDSCs, but not M-MDSCs, were expanded in patients with T2DN, although stage of chronic kidney disease and levels of eGFR and uPCR did not correlate with this expansion.
Figure 2. Correlation between MDSCs and kidney parameters: eGFR (A-C) and random uPCR (D-F). Correlation of eGFR with (A) M-MDSCs, (B) PMN-MDSCs, and (C) Lox-1+ PMN-MDSCs. Correlation of uPCR with (D) M-MDSCs, (E) PMN-MDSCs, and (F) Lox-1+ PMN-MDSCs.
ROS production in PMN-MDSC
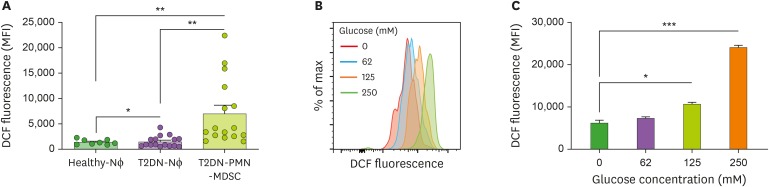
As a representative function of PMN-MDSC, we evaluated the production of ROS (18). ROS production was higher in PMN-MDSCs from T2DN patients than in neutrophils from both patients and healthy individuals (Fig. 3A). When PMN-MDSCs from patients with T2DN were cultured with high concentrations of glucose, ROS production was further augmented depending on the glucose concentrations (Fig. 3B and C). Collectively, ROS production in PMN-MDSCs of T2DN patients was elevated compared with neutrophils, and this might be enhanced by the diabetic condition.
Figure 3. Production of ROS. (A) Comparison of ROS production between neutrophils and PMN-MDSC from patients with T2DN and neutrophils from healthy individuals. (B) Representative histogram and (C) bar graph for DCF fluorescence in cultured PMN-MDSCs with indicated glucose concentrations.
Nɸ, neutrophil; MFI, mean fluorescence intensity; DCF, dichlorofluorescein.
*p<0.05; **p<0.01; ***p<0.001.
Effect of PMN-MDSCs on renal progression
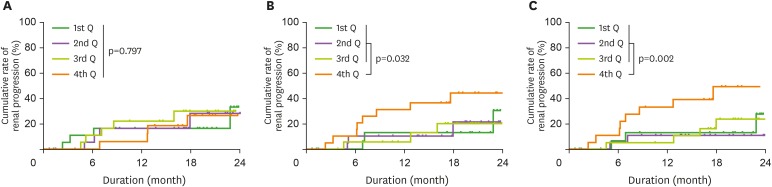
Renal progression occurred in 20 patients during the median period of 17 months (7–20 months; maximum 2 years). The progression group had higher levels of serum creatinine and uPCR, but lower levels of eGFR, glucose, and hemoglobin A1c than the non-progression group (Table 1). The medications used did not differ between the 2 groups. In terms of immune cell subsets, the proportion of total or Lox-1+ PMN-MDSCs was higher in the progression group than in the non-progression group. The proportion of B cells was also different between the 2 groups. To calculate hazard ratios for renal progression, we divided patients into quartiles of each MDSC subset. Fig. 4 shows Kaplan-Meier curves of renal progression according to the quartiles of MDSCs. The 4 quartile groups of M-MDSC had a similar rate of renal progression (Fig. 4A), but the 4th quartile group of PMN-MDSC had a higher rate of renal progression than the lower 3 quartile groups (Fig. 4B). This trend was similar to the results for the quartile groups of Lox-1+ PMN-MDSC (Fig. 4C). To identify independent relationships, stepwise multivariate Cox models were applied (Table 2). High levels of PMN-MDSCs seemed to correlate with the risk of renal progression independently of other variables, although the significances were marginal. Lox-1+ PMN-MDSCs had a more independent relationship with renal progression than PMN-MDSCs. Levels of M-MDSC were not associated with renal progression.
Figure 4. Cumulative rate of renal progression according to the quartiles of (A) monocytic myeloid-derived suppressor cells, (B) polymorphonuclear myeloid-derived suppressor cells, (C) Lox-1+ polymorphonuclear myeloid-derived suppressor cells.
Q, quartile.
Table 2. Renal progression-predicting model with MDSCs.
| Variables | Quartiles | Model 1 | Model 2 | Model 3 | Model 4 | ||||
|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | ||
| M-MDSCs | 1st Q | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) | ||||
| 2nd Q | 1.05 (0.26–4.20) | 0.949 | 0.83 (0.20–3.54) | 0.804 | 1.01 (0.16–6.21) | 0.994 | 0.73 (0.128–4.22) | 0.729 | |
| 3rd Q | 1.64 (0.46–5.82) | 0.446 | 1.30 (0.36–4.79) | 0.689 | 1.22 (0.28–5.43) | 0.793 | 1.95 (0.35–10.99) | 0.451 | |
| 4th Q | 1.59 (0.45–5.64) | 0.477 | 1.90 (0.51–7.13) | 0.340 | 1.63 (0.35–7.63) | 0.534 | 1.41 (0.26–7.52) | 0.690 | |
| PMN-MDSCs | 1st Q | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) | ||||
| 2nd Q | 0.96 (0.19–4.79) | 0.963 | 0.74 (0.14–3.96) | 0.726 | 0.83 (0.13–5.21) | 0.840 | 0.83 (0.12–6.04) | 0.857 | |
| 3rd Q | 1.69 (0.40–7.12) | 0.472 | 1.02 (0.23–4.44) | 0.983 | 0.82 (0.14–4.95) | 0.827 | 1.08 (0.20–6.00) | 0.927 | |
| 4th Q | 3.07 (0.83–11.37) | 0.093 | 4.40 (1.07–18.06) | 0.040 | 4.02 (0.84–19.29) | 0.082 | 5.95 (0.97–36.61) | 0.054 | |
| Lox-1+ PMN-MDSCs | 1st Q | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) | ||||
| 2nd Q | 0.71 (0.12–4.29) | 0.708 | 0.55 (0.09–3.39) | 0.521 | 0.44 (0.07–2.86) | 0.391 | 0.51 (0.05–5.24) | 0.567 | |
| 3rd Q | 1.49 (0.35–6.28) | 0.587 | 1.28 (0.28–5.92) | 0.749 | 3.71 (0.48–28.66) | 0.209 | 3.11 (0.43–22.27) | 0.259 | |
| 4th Q | 4.03 (1.09–14.93) | 0.037 | 6.85 (1.54–30.38) | 0.011 | 12.32 (1.78–85.24) | 0.011 | 37.69 (2.45–579.44) | 0.009 | |
Model 1: Unadjusted. Model 2: Adjusted for age, sex, and eGFR. Model 3: Adjusted for other clinical and laboratory variables plus Model 2. Model 4: Adjusted for other immune cell subset plus Model 2.
HR, hazard ratio; CI, confidence interval; Q, quartile.
DISCUSSION
T2DN is regarded as an inflammatory disease, and renal function is aggravated by inflammatory insults. However, this inflammatory milieu generated by several immune cell subsets in T2DN has not been fully explored. The present study focused on MDSCs and identified that PMN-MDSCs were primarily expanded in patients with T2DN and their ROS production as a functional capacity was potentially maintained and augmented by the diabetic condition. Higher expansions of total and Lox-1+ PMN-MDSCs were associated with worse renal outcome than less expanded counterparts, which suggests that PMN-MDSCs could be an immune biomarker or a therapeutic target in T2DN.
MDSCs are one type of immunosuppressive cell (19). These cells are frequently expanded in tumor conditions and their unique functions, such as production of ROS, arginase, nitric oxide, and anti-inflammatory cytokines, and the expression of inhibitory immune checkpoint molecules promote tumor progression (11). Interestingly, the presence of MDSCs has been documented in several non-tumorous conditions such as autoimmune or chronic inflammatory diseases (12,13). Inflamed tissues may attract the migration of MDSCs although the linking molecules have not been comprehensively identified (20). In accordance with these observations, a preclinical model under diabetic condition displays an enriched MDSC subset (14), and thus, suggests MDSCs could be a therapeutic tool (21). Diabetic patients have shown an expansion of MDSC (14), but this issue has been documented primarily in type 1 diabetes. One study focused on 24 patients with type 2 diabetes, but these patients did not receive oral anti-diabetic drugs (i.e., taking insulin alone) and did not have T2DN, and thus, may not be representative of patients with treated type 2 diabetes or T2DN (22). Furthermore, that study did not differentiate target cells into M-MDSCs and PMN-MDSCs in the analyses. The indicated proportion of MDSCs seemed to be approximately 6%–7% (the study did not report the exact proportion), and this was similar to the findings in the present study (median, 6.7% [3.9%–12.3%]). The present study further identified that 96% of MDSCs were the PMN-subset; and 78% of PMN-MDSCs expressed Lox-1. Their functional capacity, illustrated using ROS production, was augmented by hyperglycemic conditions. These results have clinical implications because therapeutic targeting can be further focused on PMN-MDSCs in T2DN.
Preclinical models have found that MDSCs regulate the severity of kidney injury. In acute renal inflammatory models such as ischemia-reperfusion injury (23) and doxorubicin-induced glomerulonephritis (24), MDSCs were expanded in both blood and injured kidneys and their adoptive transfer reduced renal damage. The expansion of MDSCs was also identified in a chronic fibrosis model using adenine (25), and renal fibrosis was reduced by the infusion of MDSCs in a streptozotocin-induced DN model (26). One human study of 49 patients with end-stage renal disease found that PMN-MDSCs were primarily elevated in these patients and the M-MDSC subset was intriguingly elevated after a session of hemodialysis alone, although the underlying mechanism was not provided (27). The present study increases the knowledge of MDSCs in kidney diseases, specifically in T2DN, which is the most common cause of end-stage renal disease (1).
The presence or expansion of MDSCs has frequently been associated with poorer outcomes of patients with cancer (28). In the present study, high expansion of PMN-MDSCs correlated with subsequent renal progression, although the increase in MDSCs was not dependent on the stage of T2DN or other parameters of kidney dysfunction such as eGFR and uPCR. When their anti-inflammatory functions are considered, this relationship seems to be counterintuitive. Some hypotheses may be suggested regarding this reversed relationship. The anti-inflammatory capacity of PMN-MDSCs in T2DN might be insufficient to maintain kidney function, because several other inflammatory cells, cytokines, adipokines, and chemokines would participate in and aggravate the renal inflammatory milieu (9,29). Additionally, the capacity of PMN-MDSCs might be altered in T2DN compared with tumorous conditions. Particularly, uremic conditions are known to blunt the anti-inflammatory function of immune cells (30). This potential alteration of functions has been much explored in the Treg subset. The number of Tregs is infrequently elevated in the diabetic state (31,32), and their functions are reduced in chronic kidney disease irrespective of stage (33,34). To improve the anti-inflammatory function of PMN-MDSCs and make them a potential therapeutic target in T2DN, other solutions may be needed such as expansion of ex vivo or in vivo cytokine-augmented MDSCs (14,26).
Understanding of the inflammatory milieu in T2DN is essential to develop immune cell-targeting therapy for prevention of renal damage. The present study identifies the expansion of PMN-MDSCs in T2DN, and their high expansion is related to renal outcome. These results will form the basis of future studies to understand the pathophysiology of human T2DN and to develop immune cell-targeting therapy.
ACKNOWLEDGEMENTS
This study was supported by the Young Investigator Research Grant from the Korean Society Nephrology (Kyowa Hakko Kirin 2017) and a grant from the Basic Science Research Program through the National Research Foundation of Korea (NRF), which is funded by the Ministry of Education (NRF-2017R1D1A1B03031642, NRF-2015R1C1A1A01054596, and NRF-2018R1D1A1A02085326). The funders played no role in the study design, data collection, analysis, interpretation, or manuscript writing. The biospecimens were provided by the Seoul National University Hospital Human Biobank, a member of the National Biobank of Korea, which is supported by the Ministry of Health and Welfare, Republic of Korea.
Abbreviations
- DN
diabetic nephropathy
- eGFR
estimated glomerular filtration rate
- M-
monocytic
- MDSC
myeloid-derived suppressor cell
- PMN-
polymorphonuclear
- T2DN
type 2 diabetic nephropathy
- uPCR
urine protein-to-creatinine ratio
Footnotes
Conflict of Interest: The authors declare no potential conflicts of interest.
- Conceptualization: Islam J, Youn JI, Han SS.
- Data curation: Islam J, Lee HJ, Yang SH.
- Formal analysis: Islam J, Lee DS, Youn JI, Han SS.
- Resources: Kim DK, Joo KW, Kim YS.
- Supervision: Seo SU, Seong SY.
- Writing - original draft: Youn JI, Han SS.
- Writing - review & editing: Youn JI, Han SS.
SUPPLEMENTARY MATERIALS
Abs used in this study
Mean proportion of immune cell subset among CD45+ cells
Cell number of MDSCs in patients with T2DN. (A) M-MDSC. (B) PMN-MDSC. (C) Lox-1+ PMN-MDSC.
References
- 1.Locatelli F, Canaud B, Eckardt KU, Stenvinkel P, Wanner C, Zoccali C. The importance of diabetic nephropathy in current nephrological practice. Nephrol Dial Transplant. 2003;18:1716–1725. doi: 10.1093/ndt/gfg288. [DOI] [PubMed] [Google Scholar]
- 2.Lok CE, Oliver MJ, Rothwell DM, Hux JE. The growing volume of diabetes-related dialysis: a population based study. Nephrol Dial Transplant. 2004;19:3098–3103. doi: 10.1093/ndt/gfh540. [DOI] [PubMed] [Google Scholar]
- 3.Chang YT, Wu JL, Hsu CC, Wang JD, Sung JM. Diabetes and end-stage renal disease synergistically contribute to increased incidence of cardiovascular events: a nationwide follow-up study during 1998–2009. Diabetes Care. 2014;37:277–285. doi: 10.2337/dc13-0781. [DOI] [PubMed] [Google Scholar]
- 4.Afkarian M, Sachs MC, Kestenbaum B, Hirsch IB, Tuttle KR, Himmelfarb J, de Boer IH. Kidney disease and increased mortality risk in type 2 diabetes. J Am Soc Nephrol. 2013;24:302–308. doi: 10.1681/ASN.2012070718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nichols GA, Vupputuri S, Lau H. Medical care costs associated with progression of diabetic nephropathy. Diabetes Care. 2011;34:2374–2378. doi: 10.2337/dc11-0475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levin A, Stevens PE. Summary of KDIGO 2012 CKD Guideline: behind the scenes, need for guidance, and a framework for moving forward. Kidney Int. 2014;85:49–61. doi: 10.1038/ki.2013.444. [DOI] [PubMed] [Google Scholar]
- 7.Karalliedde J, Gnudi L. Diabetes mellitus, a complex and heterogeneous disease, and the role of insulin resistance as a determinant of diabetic kidney disease. Nephrol Dial Transplant. 2016;31:206–213. doi: 10.1093/ndt/gfu405. [DOI] [PubMed] [Google Scholar]
- 8.Toth-Manikowski S, Atta MG. Diabetic kidney disease: pathophysiology and therapeutic targets. J Diabetes Res. 2015;2015:697010. doi: 10.1155/2015/697010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zheng Z, Zheng F. Immune cells and inflammation in diabetic nephropathy. J Diabetes Res. 2016;2016:1841690. doi: 10.1155/2016/1841690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Condamine T, Ramachandran I, Youn JI, Gabrilovich DI. Regulation of tumor metastasis by myeloid-derived suppressor cells. Annu Rev Med. 2015;66:97–110. doi: 10.1146/annurev-med-051013-052304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gabrilovich DI. Myeloid-derived suppressor cells. Cancer Immunol Res. 2017;5:3–8. doi: 10.1158/2326-6066.CIR-16-0297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boros P, Ochando J, Zeher M. Myeloid derived suppressor cells and autoimmunity. Hum Immunol. 2016;77:631–636. doi: 10.1016/j.humimm.2016.05.024. [DOI] [PubMed] [Google Scholar]
- 13.Sendo S, Saegusa J, Morinobu A. Myeloid-derived suppressor cells in non-neoplastic inflamed organs. Inflamm Regen. 2018;38:19. doi: 10.1186/s41232-018-0076-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Whitfield-Larry F, Felton J, Buse J, Su MA. Myeloid-derived suppressor cells are increased in frequency but not maximally suppressive in peripheral blood of type 1 diabetes mellitus patients. Clin Immunol. 2014;153:156–164. doi: 10.1016/j.clim.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 15.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Condamine T, Dominguez GA, Youn JI, Kossenkov AV, Mony S, Alicea-Torres K, Tcyganov E, Hashimoto A, Nefedova Y, Lin C, et al. Lectin-type oxidized LDL receptor-1 distinguishes population of human polymorphonuclear myeloid-derived suppressor cells in cancer patients. Sci Immunol. 2016;1:aaf8943. doi: 10.1126/sciimmunol.aaf8943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nan J, Xing YF, Hu B, Tang JX, Dong HM, He YM, Ruan DY, Ye QJ, Cai JR, Ma XK, et al. Endoplasmic reticulum stress induced LOX-1+ CD15+ polymorphonuclear myeloid-derived suppressor cells in hepatocellular carcinoma. Immunology. 2018;154:144–155. doi: 10.1111/imm.12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ohl K, Tenbrock K. Reactive oxygen species as regulators of MDSC-mediated immune suppression. Front Immunol. 2018;9:2499. doi: 10.3389/fimmu.2018.02499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Youn JI, Gabrilovich DI. The biology of myeloid-derived suppressor cells: the blessing and the curse of morphological and functional heterogeneity. Eur J Immunol. 2010;40:2969–2975. doi: 10.1002/eji.201040895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheng L, Wang J, Li X, Xing Q, Du P, Su L, Wang S. Interleukin-6 induces Gr-1+CD11b+ myeloid cells to suppress CD8+ T cell-mediated liver injury in mice. PLoS One. 2011;6:e17631. doi: 10.1371/journal.pone.0017631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yin B, Ma G, Yen CY, Zhou Z, Wang GX, Divino CM, Casares S, Chen SH, Yang WC, Pan PY. Myeloid-derived suppressor cells prevent type 1 diabetes in murine models. J Immunol. 2010;185:5828–5834. doi: 10.4049/jimmunol.0903636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang T, Wen Y, Fan X. Myeloid-derived suppressor cells suppress CD4+ T cell activity and prevent the development of type 2 diabetes. Acta Biochim Biophys Sin (Shanghai) 2018;50:362–369. doi: 10.1093/abbs/gmy014. [DOI] [PubMed] [Google Scholar]
- 23.Zhang C, Wang S, Li J, Zhang W, Zheng L, Yang C, Zhu T, Rong R. The mTOR signal regulates myeloid-derived suppressor cells differentiation and immunosuppressive function in acute kidney injury. Cell Death Dis. 2017;8:e2695. doi: 10.1038/cddis.2017.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li L, Zhang T, Diao W, Jin F, Shi L, Meng J, Liu H, Zhang J, Zeng CH, Zhang MC, et al. Role of myeloid-derived suppressor cells in glucocorticoid-mediated amelioration of fsgs. J Am Soc Nephrol. 2015;26:2183–2197. doi: 10.1681/ASN.2014050468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Höchst B, Mikulec J, Baccega T, Metzger C, Welz M, Peusquens J, Tacke F, Knolle P, Kurts C, Diehl L, et al. Differential induction of Ly6G and Ly6C positive myeloid derived suppressor cells in chronic kidney and liver inflammation and fibrosis. PLoS One. 2015;10:e0119662. doi: 10.1371/journal.pone.0119662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hsieh CC, Lin CL, He JT, Chiang M, Wang Y, Tsai YC, Hung CH, Chang PJ. Administration of cytokine-induced myeloid-derived suppressor cells ameliorates renal fibrosis in diabetic mice. Stem Cell Res Ther. 2018;9:183. doi: 10.1186/s13287-018-0915-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xing YF, Cai RM, Lin Q, Ye QJ, Ren JH, Yin LH, Li X. Expansion of polymorphonuclear myeloid-derived suppressor cells in patients with end-stage renal disease may lead to infectious complications. Kidney Int. 2017;91:1236–1242. doi: 10.1016/j.kint.2016.12.015. [DOI] [PubMed] [Google Scholar]
- 28.Messmer MN, Netherby CS, Banik D, Abrams SI. Tumor-induced myeloid dysfunction and its implications for cancer immunotherapy. Cancer Immunol Immunother. 2015;64:1–13. doi: 10.1007/s00262-014-1639-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Duran-Salgado MB, Rubio-Guerra AF. Diabetic nephropathy and inflammation. World J Diabetes. 2014;5:393–398. doi: 10.4239/wjd.v5.i3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vaziri ND, Pahl MV, Crum A, Norris K. Effect of uremia on structure and function of immune system. J Ren Nutr. 2012;22:149–156. doi: 10.1053/j.jrn.2011.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qiao YC, Shen J, He L, Hong XZ, Tian F, Pan YH, Liang L, Zhang XX, Zhao HL. Changes of regulatory T cells and of proinflammatory and immunosuppressive cytokines in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. J Diabetes Res. 2016;2016:3694957. doi: 10.1155/2016/3694957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Viisanen T, Gazali AM, Ihantola EL, Ekman I, Näntö-Salonen K, Veijola R, Toppari J, Knip M, Ilonen J, Kinnunen T. Foxp3+ regulatory T cell compartment is altered in children with newly diagnosed type 1 diabetes but not in autoantibody-positive at-risk children. Front Immunol. 2019;10:19. doi: 10.3389/fimmu.2019.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meier P, Golshayan D, Blanc E, Pascual M, Burnier M. Oxidized LDL modulates apoptosis of regulatory T cells in patients with ESRD. J Am Soc Nephrol. 2009;20:1368–1384. doi: 10.1681/ASN.2008070734. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 34.Hendrikx TK, van Gurp EA, Mol WM, Schoordijk W, Sewgobind VD, Ijzermans JN, Weimar W, Baan CC. End-stage renal failure and regulatory activities of CD4+CD25bright+FoxP3+ T-cells. Nephrol Dial Transplant. 2009;24:1969–1978. doi: 10.1093/ndt/gfp005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Abs used in this study
Mean proportion of immune cell subset among CD45+ cells
Cell number of MDSCs in patients with T2DN. (A) M-MDSC. (B) PMN-MDSC. (C) Lox-1+ PMN-MDSC.