Figure 5.
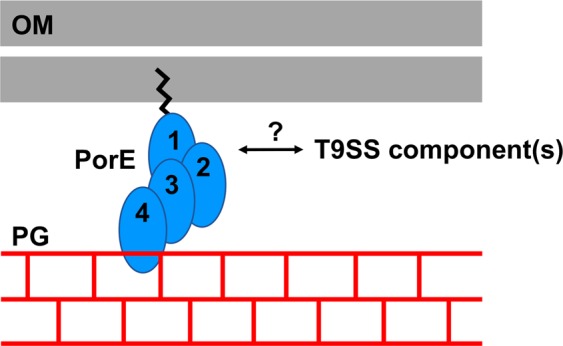
Schematic representation of the putative role of PorE in anchoring T9SS to the bacterial cell wall. PorE is a lipoprotein composed of four domains (1: tetratricopeptide repeat domain (TPR), 2: β–propeller domain (WD40), 3: carboxypeptidase regulatory domain-like fold (CRD), and 4: OmpA_C-like putative peptidoglycan-binding domain). Therefore, PorE is anchored to the inner leaflet of the OM through its N-terminal acylation, and could interact with the peptidoglycan network through the C-terminal OmpA_C-like domain. Interaction with (a) putative T9SS component(s) could be mediated by the TPR and WD40 domains that are structural scaffolds often involved in protein-protein interactions. The figure was prepared using MICROSOFT PowerPoint (version 16.16.20).
