FIGURE 7.
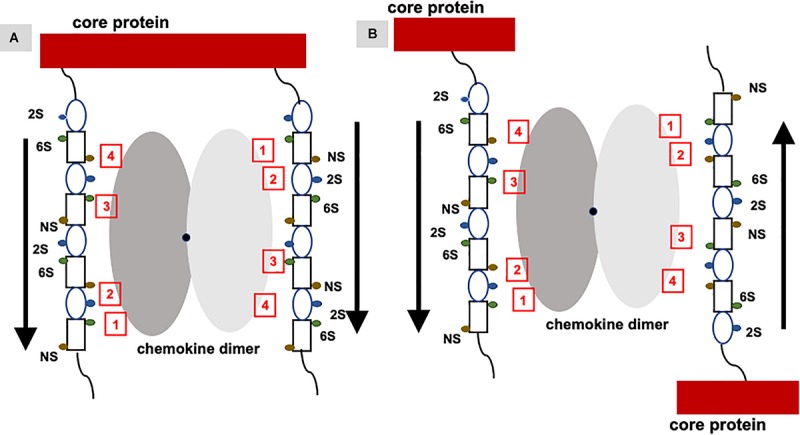
Binding of a chemokine dimer to PG GAGs. Monomers of the chemokine dimer are shown in different shades of gray. For illustrative purposes, GAG-binding residues in each monomer of the chemokine dimer are labeled 1 to 4, which run in opposite directions because of two-fold symmetry about the dimer interface (shown by a black dot). Schematic of an heparin octasaccharide with iduronate and glucosamine shown as an oval and rectangle, and N-sulfate (NS), 2-sulfate (2S), and 6-sulfate (6S) shown as spheres in different colors. Helical structure of heparin clusters the 3 sulfate groups on opposite faces of the helical axis (44). (A) Binding of a chemokine dimer to two GAG chains within a PG. Direction of the GAG orientation are shown by arrows. The schematic shows GAG interactions of the two monomers cannot be the same, and that binding to both GAG chains is only possible if the binding interactions of the two monomers are different. (B) Binding of a chemokine to two GAG chains from two PGs. Direction of the GAG orientation are shown by arrows. In this case, two monomers of the dimer can encounter GAG chains running in opposite directions and so similar interactions to both monomers are possible. Such binding could occur in PCM and ECM due to some degree of motional freedom of the PGs.
