Fig. 1.
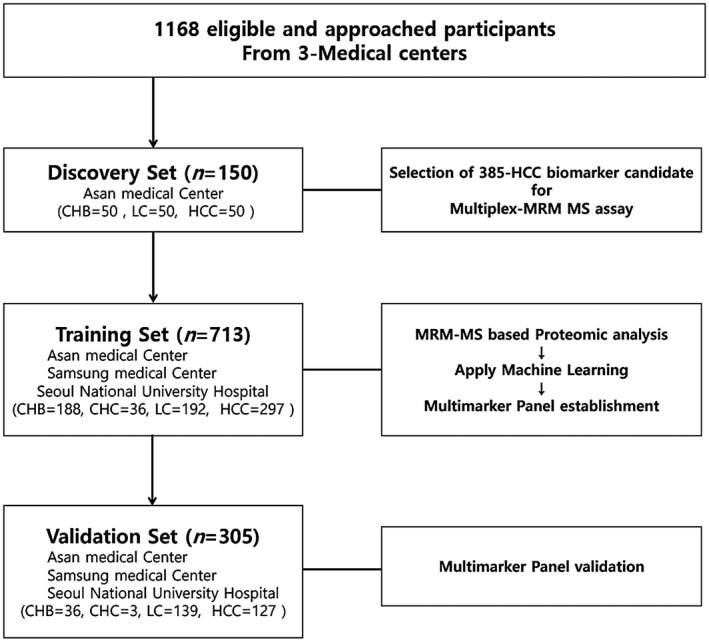
Overview of the workflow. Patients were enrolled in independent discovery, training, and validation sets. The 385 HCC biomarker candidates were identified by an MRM‐MS‐based proteomic method in the discovery set, consisting of serum samples from 50 patients with early HCC and 100 high‐risk controls (50 with CHB and 50 with LC). The multimarker panel that was established in the training set consisted of 713 serum samples, consisting of 297 patients with HCC and 416 high‐risk controls (187 with CHB, 36 with CHC, and 193 with LC) enrolled from three sites. The validation set included 127 patients with HCC and 178 high‐risk controls (36 with CHB, 3 with CHC, and 139 with LC) enrolled from the three aforementioned sites and was used to further evaluate the performance of the multimarker panel.
