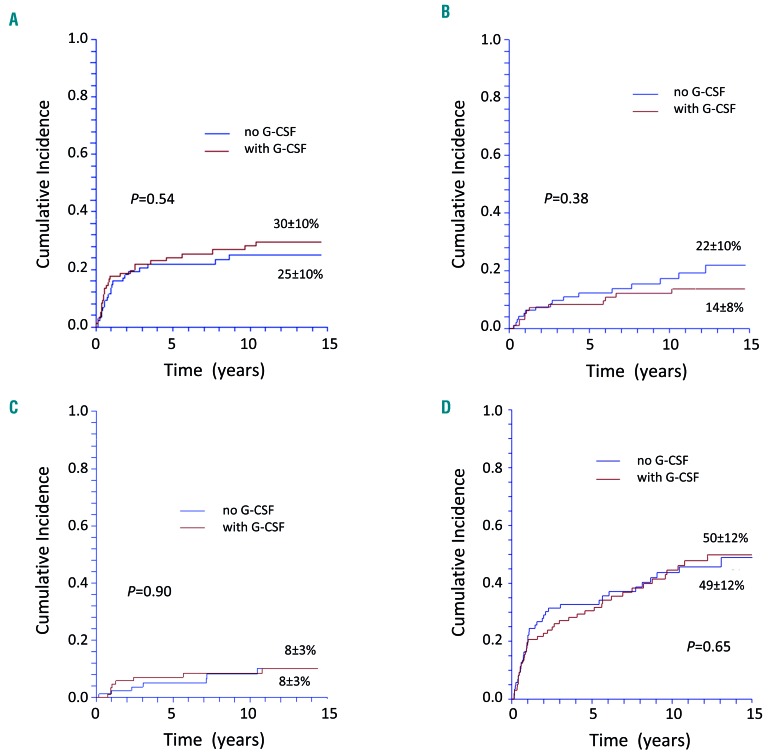
Figure 3.
Cumulative incidence of late complications of patients with severe aplastic anemia treated with horse antithymocyte globulin and cyclosporine with or without granulocyte colony-stimulating factor. (A) Cumulative incidence of relapse of patients with severe aplastic anemia (SAA) treated with horse antithymocyte globulin (ATG) and cyclosporine (CSA) with or without granulocyte colony-stimulating factor (G-CSF). (B) Cumulative probability of being transplanted (death without transplant is the competing event) of patients with SAA treated in first-line with horse ATG and CSA with or without G-CSF. (C) Cumulative incidence of clonal evolution to a hematologic malignancy (myelodysplastic syndrome/acute myeloid leukemia or isolated cytogenetic abnormality) of patients with SAA treated with horse ATG and CSA with or without G-CSF. (D) Cumulative incidence of any late event (relapse, myelodysplastic syndrome/acute myeloid leukemia, isolated cytogenetic abnormality, solid cancer, clinical paroxysmal nocturnal hemoglobinuria, aseptic osteonecrosis, chronic kidney disease) of patients with SAA treated with horse ATG and CSA with or without G-CSF.