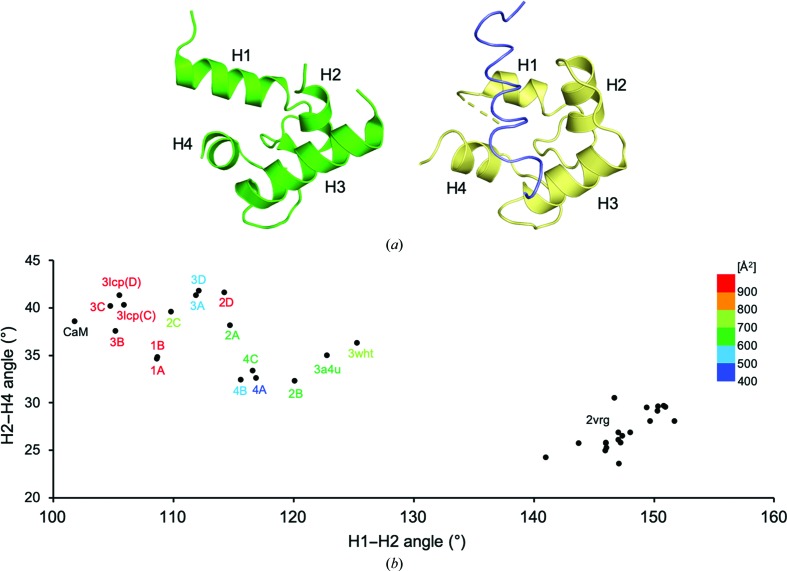
Figure 2.
Conformational variations of the ligand-binding site of MCFD2. (a) Relative positions of helices H1–H4 in the EF-hand proteins MCFD2 and calmodulin. Structures of MCFD2 in complex with ERGIC-53CRD (PDB entry 3a4u; Nishio et al., 2010 ▸) and the ligand-bound calmodulin complex (PDB entry 1cff; Elshorst et al., 1999 ▸) are shown on the left and right, respectively. The calmodulin and Ca2+-pump peptide ligand structures are colored yellow and slate, respectively. (b) Correlative helix-angle distributions in the ligand-binding site of MCFD2. Vertical and horizontal axes indicate H2–H4 and H1–H2 helix angles, respectively. The data represent helix-angle conformations derived from currently determined MCFD2 structures together with previously reported MCFD2 and calmodulin structures, which are labeled with ‘complex name’, ‘PDB code’ or ‘CaM’, respectively, with a color gradient according to their crystal contact areas. For the apo MCMD2 NMR structure (PDB entry 2vrg; Guy et al., 2008 ▸), the angles derived from the 20 lowest target-function structures are shown.