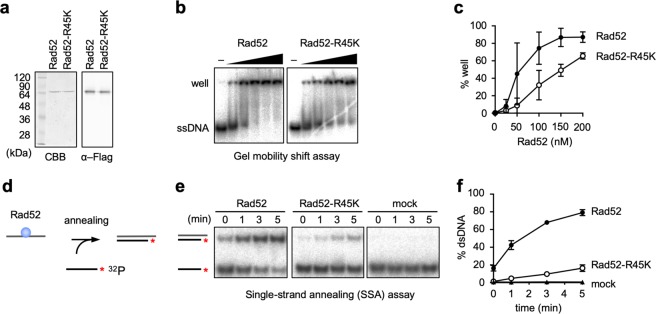
Fig. 4. The rad52-R45K mutation impairs the SSA activity of the Rad52 protein.
a Purified Rad52 and Rad52-R45K proteins were separated by 12% SDS-PAGE and stained with CBB or immunostained using anti-Flag antibody (α–Flag). b Gel mobility shift assays. 32P-labelled Oligo211 (48 nt, 2 nM in DNA molecules) and Rad52 (0, 25, 50, 100, 150, and 200 nM) were incubated at 30 °C for 10 min. After addition of loading buffer, the mixture was resolved by 10% non-denaturing PAGE. c Percentages of well signals in whole-lane signals. The mean and s.d. of three independent experiments are shown. d Scheme of Rad52-mediated SSA assays. The asterisk represents the 32P-label. e SSA assays. After incubating Rad52 (1.35 nM) with Oligo508 (53 nt, 0.4 nM in DNA molecules) for 10 min at 30 °C, 32P-labelled Oligo211 (48 nt, 0.3 nM in DNA molecules) was added. After the indicated periods of time, DNAs were purified and resolved by 10% non-denaturing PAGE. f Percentages of dsDNA signals in whole-lane signals. Uncropped images of the gels presented in b, e are shown in Supplementary Fig. 8. Source data for the graphs in c, f are available in Supplementary Data 1.