Figure 1. Trial Design.
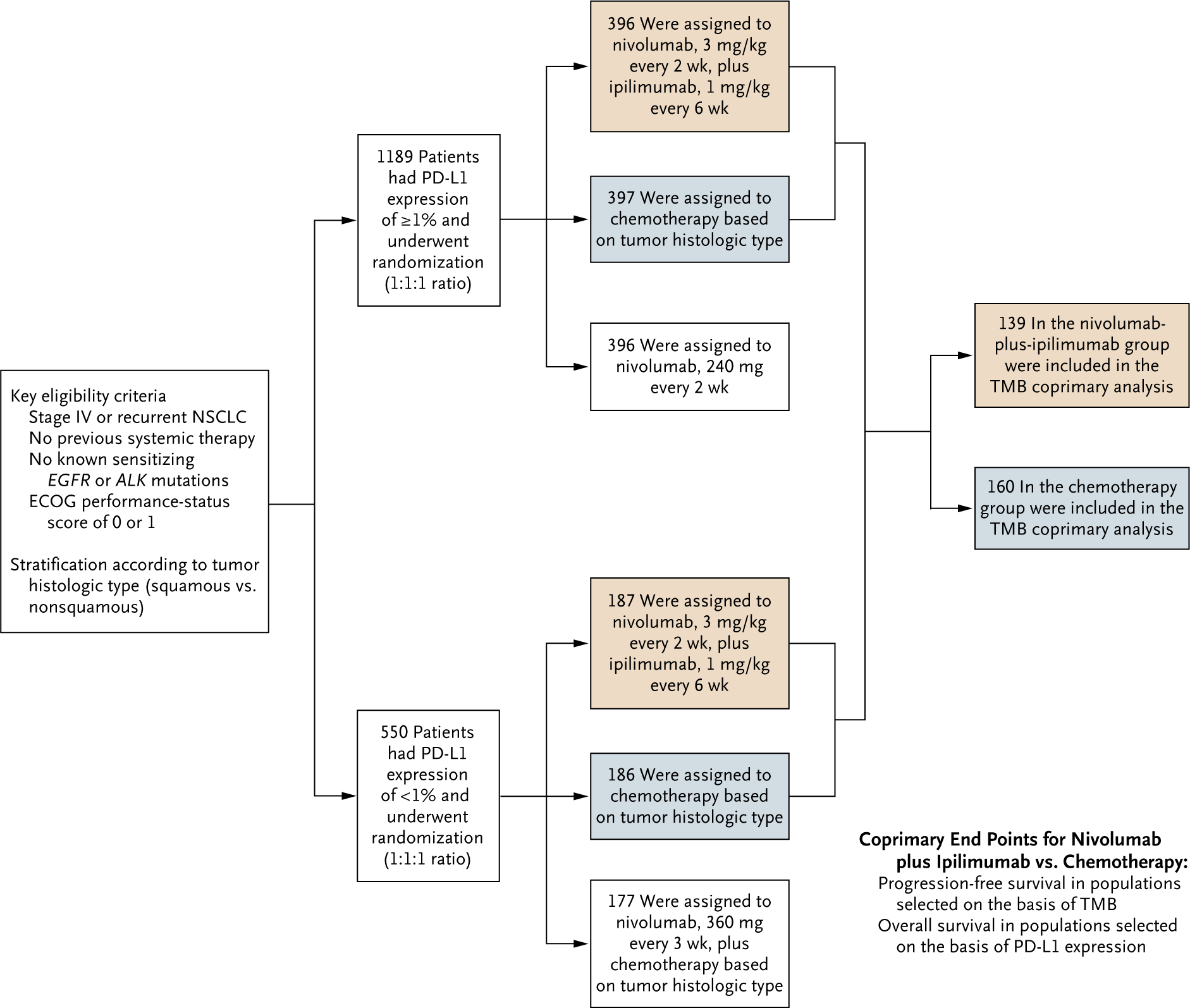
Chemotherapy for patients with nonsquamous non–small-cell lung cancer (NSCLC) consisted of pemetrexed (500 mg per square meter of body-surface area) plus cisplatin (75 mg per square meter) or carboplatin (area under the concentration-time curve [AUC], 5 or 6), every 3 weeks for up to four cycles, with optional maintenance therapy with pemetrexed (500 mg per square meter) after chemotherapy or with nivolumab (360 mg every 3 weeks) plus pemetrexed (500 mg per square meter) after nivolumab plus chemotherapy. Chemotherapy for patients with squamous NSCLC consisted of gemcitabine (1000 or 1250 mg per square meter) plus cisplatin (75 mg per square meter), or gemcitabine (1000 mg per square meter) plus carboplatin (AUC, 5), every 3 weeks for up to four cycles. The tumor mutational burden (TMB) coprimary analysis was conducted in the subgroup of patients assigned to nivolumab plus ipilimumab or chemotherapy who had a TMB of at least 10 mutations per megabase. Given the recommendation of the data and safety monitoring committee to continue the trial for overall survival, analysis of the coprimary end point of overall survival among patients selected on the basis of the programmed death ligand 1 (PD-L1) expression level was not performed for the current database lock. Eastern Cooperative Oncology Group (ECOG) performance-status scores range from 0 to 5, with higher scores indicating greater disability.
