Abstract
Duchene Muscular Dystrophy (DMD) is an X-linked recessive genetic disease caused by a lack of functional dystrophin protein. The disease cannot be cured, and as the disease progresses, the patient develops symptoms of dilated cardiomyopathy, arrhythmia, and congestive heart failure. The DMDMDX mutant mice do not express dystrophin, and are commonly used as a mouse model of DMD. In our recent study, we observed that intramyocardial injection of wide type (WT)-myogenic progenitor cells-derived exosomes (MPC-Exo) transiently restored the expression of dystrophin in the myocardium of DMDMDX mutant mice, which was associated with a transient improvement in cardiac function suggesting that WT-MPC-Exo may provide an option to relieve the cardiac symptoms of DMD. This article describes the technique of MPC-Exo purification and transplantation into hearts of DMDMDX mutant mice.
Keywords: Genetics, Issue 146, Exosome, Duchenne muscular dystrophy, Myogenic progenitor cell, sequential ultracentrifugation, Exosome Transplantation, echocardiography, heart function
Introduction
Duchenne muscular dystrophy (DMD) is an X-linked recessive, progressive neuromuscular disease caused by a mutation in DMD gene and the loss of functional dystrophin1. Dystrophin is expressed primarily in skeletal muscle and myocardium, and is less expressed in smooth muscle, endocrine glands and neurons2,3. DMD is the most common type of muscular dystrophy with an incidence of one per 3,500 to 5,000 newborn boys worldwide4,5. Individuals typically develop progressive muscle necrosis, loss of independent walking by early adolescence, and death in the second to third decades of their lives due to heart failure and respiratory failure6.
Dilated cardiomyopathy, arrhythmias and congestive heart failure are common cardiovascular manifestations of DMD7,8. The disease can’t be cured, supportive treatment may improve symptoms or delay the progression of heart failure, but it is very difficult to improve the heart function9,10.
Similar to DMD patients, X-linked muscular dystrophy (MDX) mice are deficient in dystrophin protein and present symptoms of cardiomyopathy11, and are therefore widely used in DMD associated cardiomyopathy research. In order to restore dystrophin in affected muscles, allogeneic stem cell therapy has proven to be an effective treatment for DMD12,13,14. Exosomes, 30-150 nm membrane vesicles secreted by various cell types play a key role in cell-to-cell communication through genetic material transport, such as messenger RNA (mRNA) and non-coding RNAs15,16,17,18,19,20,21.
Our previous studies have shown that exosomes derived from myogenic progenitor cells (MPC), such as C2C12 cell line, can transfer dystrophin mRNA to host cardiomyocytes after direct cardiac injection22, indicating that allogeneic delivery of MPC-derived exosomes (MPC-Exo) can transiently restore DMD gene expression in MDX mice. This article focuses on MPC-Exo purification and transplantation techniques.
Protocol
Animals were handled according to approved protocols and animal welfare regulations of the Institutional Animal Care and Use Committee of the Medical College of Georgia at Augusta University.
1. Isolation and Purification of MPC-derived Exosomes
Seed 5 × 106 C2C12 cells in a 15 cm cell culture dish with 20 mL complete Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum (FBS), 100 U/mL penicillin G and 100 μg/mL streptomycin. Incubate at 37 °C and 50% CO2.
Prepare exosome-depleted medium by ultracentrifugation of FBS at 100,000 x g for 18 h at 4 °C using a swinging bucket rotor. Discard the pellet.
Replace the complete DMEM with exosome-depleted medium when monolayer cells reach 80% confluence in the culture dish.
Use a transfer pipette to collect the supernatant from the cell culture dish every 48 h for a total of three times. Collect the supernatant in 50 mL centrifuge tubes, and centrifuge at 150 x g for 10 min to remove the remaining cells.
Filter the supernatant through a 0.22 μm filter to eliminate cell debris. Ultracentrifuge the filtered medium in ultra-clear tubes using the swinging bucket rotor at 100,000 x g for 120 min at 4 °C to precipitate exosomes.
Resuspend the exosome-containing pellet in phosphate buffered saline (PBS) and fill the entire ultra-dear tube with PBS. Perform ultracentrifugation of this suspension at 100,000 x g for 120 min at 4 °C to eliminate contaminating proteins.
Discard the supernatant and resuspend the exosome pellet in 100 μL PBS. Store at −80 °C for future use.
For electron microscopy analysis, place 3 μL of exosomal suspension on a 200 mesh formvar-coated copper electron microscope grid for 5 min at room temperature. Continue with standard uranyl acetate staining23. Examine the semi-dry grid by transmission electron microscopy.
2. Intramyocardial Exosome Delivery
NOTE: We used six mice in each group.
Anesthetize DBA/2J-DMDMDX mice (male; age > 10 months; body weight (BW) > 18 g) with intraperitoneal (i.p.) injection of 100 mg/kg BW of ketamine combined with 10 mg/kg BW of xylazine, and confirm the depth of anesthesia by toe pinch.
Fix each mouse in supine position on a surgical platform by using tape to secure each limb and place a 3-0 suture horizontally below the upper teeth to hold the upper jaw in place.
Apply the depilatory cream to the left side of the mouse chest to remove the fur from the skin.
Perform endotracheal intubation via oral cavity using a 24 G catheter, and ventilate the mouse with room air at a rate of 195 breaths/min using a rodent ventilator (see the Table of Materials).
Disinfect the skin with 75% alcohol and 10% povidone iodine.
Make a 10–15 mm oblique incision from the left sternal edge to the left armpit, cut the pectoralis major and pectoralis minor by a scissor, then make a left thoracotomy through the fourth intercostal space. Gently insert the retractor bands to spread the thoracic cavity to a width of 10 mm. Take care to avoid damage to the left lung.
Use two straight tweezers to remove the pericardium, pull them apart, and place them behind the retractor tips to expose the heart. Inject MPC-Exo (50 μg in 30 μL PBS) or 30 μL PBS (as a control) intramyocardially into the anterior wall of the left ventricle using a 31 G insulin needle at one site.
Use 6-0 nylon sutures to close the thoracic cavity, pectoralis muscles and skin in sequence.
Recover mice. Once rhythmic, spontaneous breathing is present, remove the mice from the ventilator, and extubate. Observe and record the mice condition after surgery.
Materials
| Name | Company | Catalog Number | Comments |
|---|---|---|---|
| 0.22 μm Filter | Fisherbrand | 09-720-004 | |
| 15 cm Cell Culture Dish | Thermo Fisher Scientific | 157150 | |
| 24 G catheter | TERUMO | SR-OX2419CA | |
| 31 G insulin needle | BD | 328291 | |
| 4% paraformaldehyde | Affymetrix | AAJ19943K2 | |
| 50 mL Centrifuge Tubes | Thermo Fisher Scientific | 339652 | |
| 6-0 suture | Pro Advantage by NDC | P420697 | |
| Alexa Fluor 488 goat anti-rabbit IgG | Thermo Fisher Scientific | A-11008 | |
| Antibiotic Antimycotic Solution | Corning | 30-004-CI | |
| Anti-Dystrophin antibody | Abcam | ab15277 | |
| Antigen retriever | Aptum Biologics | R2100-US | Antigen recovery |
| Autofluorescence Quenching Kit | Vector Laboratories | SP-8400 | |
| C2C12 cell line | ATCC | CRL-1772 | |
| Centrifuge | Unico | C8606 | |
| Change-A-Tip High Temp Cauteries | Bovie Medical Corporation | HIT | |
| Confocal microscopy | Zeiss | Zeiss 780 Upright Confocal | |
| DBA/2J-mdx mice | The Jackson Laboratory | 013141 | |
| DMEM | Corning | 10-013-CM | |
| Fetal Bovine Serum (FBS) | Corning | 35-011-CV | |
| Goat serum | MP Biomedicals, LLC | 191356 | |
| Isoflurane | Patterson Veterinary | 07-893-1389 | |
| Ketamine | Henry Schein | 056344 | |
| Mounting Medium with DAPI | Vector Laboratories | H-1500 | |
| Mouse Retractor Set | Kent Scientific | SURGI-5001 | |
| Polyethylene glycol tert-octylphenyl ether | Fisher Scientific | BP151-100 | |
| Rodent ventilator | Harvard Apparatus | 55-7066 | |
| SW-28 Ti rotor | Beckman | 342207 | |
| The Vevo 2100 Imaging Platform | FUJIFILM VisualSonics | Vevo 2100 | Ultrasound System |
| Ultracentrifuge | Beckman | 365672 | |
| Ultra-Clear Tubes | Beckman | 344058 | |
| Xylazine (XylaMed) | Bimeda-MTC Animal Health Inc. | 1XYL003 8XYL006 |
3. Echocardiography
NOTE: A single observer blinded to the experimental groups performs echocardiography and data analysis.
Two days after PBS/exosome transplantation, anesthetize the mice with 1–2% isoflurane.
Fix a mouse in supine position using tape, and apply the preheated acoustic gel on the left chest area.
- Assess left ventricular function by echocardiography as previously described16,24.
- Obtain the parasternal long axis view of the left ventricle (LV) in two dimensions (B mode), and then rotate the ultrasound probe 90° to obtain a LV short-axis view at papillary muscle level.
-
Record M-mode echocardiographic images and measure left ventricular end-diastolic diameter (LVEDD), left ventricular end-systolic volume (LVESD), left ventricular end-diastolic volume (LVEDV), and left ventricular end-systolic volume (LVESV).NOTE: Fractional shortening (FS%) = [(LVEDD - LVESD) / LVEDD] x 100, and left ventricular ejection fraction (LVEF%) = [(LVEDV - LVESV) / LVEDV] x 100.
4. Immunohistochemistry
Two days after PBS/exosome transplantation, anesthetize mice (see step 2.1) and cut the sternum open to expose the thoracic cavity. Dissect the inferior vena cava, and perfuse the heart through the left ventricle apex with 3 mL PBS, followed by 3 mL of 4% paraformaldehyde at room temperature, using a butterfly catheter with a 25 G needle attached to a 5 mL syringe.
Embed fixed hearts into paraffin, and cut into 5 μm thick sections.
Deparaffinize slides in xylene and rehydrate in ethanol solutions in following order: 100% xylene (3 min), 100% xylene (3 min), 100% ethanol (1 min), 100% ethanol (1 min), 95% ethanol (1 min), 80% ethanol (1 min), H2O (1 min).
Fix tissue sections with 4% paraformaldehyde for 8 min at room temperature.
Immerse slides in sodium citrate (0.01 M, pH 6.0) and perform antigen retrieval using an antigen retriever.
Permeabilize sections for 1 h at room temperature with 1% polyethylene glycol tert-octylphenyl ether in PBS.
Block sections with 5% goat serum in PBS for 1 h at room temperature.
Incubate sections with diluted primary antibody (anti-dystrophin antibody) in PBS (1:100) overnight at 4 °C, then wash three times with PBS for 5 min.
Incubate sections with diluted secondary antibody (Alexa Fluor 488 conjugated goat anti-rabbit IgG) in PBS (1:400) for 45 min at room temperature.
Control autofluorescence using an autofluorescence quenching kit (see the Table of Materials).
Use mounting medium containing DAPI (see the Table of Materials) to mount the slides.
Observe slides under a confocal microscope.
Representative Results
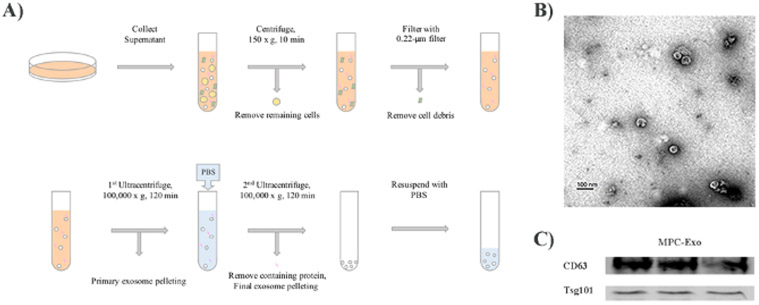
A flow chart for isolating and purifying exosomes from C2C12 cells is shown in Figure 1A. To confirm the presence of exosomes, we performed transmission electron microscopy analysis. Transmission electron microscopy image (Figure 1B) shows the morphology of the bright and round shape vesicles of C2C12 derived exosomes. Western blot analysis confirmed the presence of exosome markers, including CD63 and TSG101 (Figure 1C).
Figure 1: Exosome purification and characterization by electron microscopy.
(A) The diagram shows each step of isolation and purification of MPC-Exo. (B) The image shows round and cup-shaped vesicles under electron microscopy. Scale bar = 100 nm. (C) Western blot analysis confirmed the presence of exosome markers CD63 and TSG101.
We observed a translucent edema area after intramyocardial PBS/MPC-Exo injection into the anterior wall of the left ventricle of MDX mice (Figure 2), indicating the successful injection into the myocardium.
Figure 2: Intramyocardial injection of PBS or MPC-Exo.
A translucent edema was observed in the left ventricular anterior wall after intramyocardial PBS/exosome delivery. (A) PBS injection. (B) MPC-Exo injection.
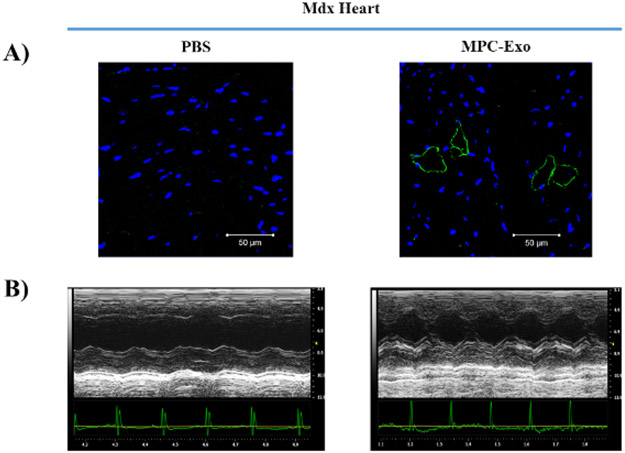
To determine whether cardiac MPC-Exo delivery restores dystrophin protein expression in MDX hearts, we performed immunofluorescent staining for dystrophin, and imaging with a confocal microscope. We observed partial restoration of dystrophin expression with membrane localization in some of cardiomyocytes (Figure 3A). To determine whether transplantation of MPC-Exo improves the cardiac function in MDX mice, we measured cardiac function by echocardiography 2 days after intramyocardial PBS / MPC-Exo delivery. As shown in Figure 3B, MPC-Exo treatment improved anterior wall movement compared with PBS, suggesting that MPC-Exo transplantation improved cardiac function in MDX mice.
Figure 3: Intramyocardial delivery of MPC-Exo partially recovers dystrophin expression in MDX hearts, and improves heart function measured by echocardiography.
(A) Confocal immunofluorescent staining the heart sections of MDX mice 2 days after intramyocardial PBS/ MPC-Exo delivery. Blue = DAPI, Green = anti-dystrophin antibody. (B) Echocardiographic measurements of cardiac function after 2 days of PBS/ MPC-Exo treatment.
Discussion
The method of isolating pure exosomes is essential for studying the function of exosomes. One of the common techniques for exosome isolation is polyethylene glycols (PEGs) mediated precipitation17,18,25. Exosomes can be precipitated in PEGs, and pelleted by low-speed centrifugation. PEG-mediated purification is very convenient, low-cost, it does not need any advanced equipment, but there is concern about the purity of exosomes since other lipoproteins may be precipitated together and are difficult to remove. Ultrafiltration is a routine method for exosome separation, based upon the molecular weight and exclusion size limits26. Ultrafiltration isolation is faster than ultracentrifugation based separation, but it can cause structural damage to large vesicles. The presence of various epitopes on the membrane of exosomes, such as CD9, CD63 and CD8127, provides an alternative method of isolating exosomes by immunoaffinity interactions between these epitopes and antibodies bound to magnetic beads. Although immunoisolation has a high specificity28, this technique has disadvantages of only isolating a subset of the total exosome population and may distort the results of the experiment. Therefore, ultracentrifugation seems to be more suitable for this study on exosome function.
In this paper, we present a method for exosomes purification by sequential ultracentrifugation. After removing the remaining cells from the supernatant by centrifugation at 150 x g, the cell debris, apoptotic bodies and vesicles larger than 220 nm were removed by passing the supernatant through a 0.22 μm filter. Exosomes were then pelleted at 100,000 x g by initial ultracentrifugation. To remove possible protein contamination, we performed a second 100,000 x g ultracentrifugation after resuspending the exosome pellet in PBS. As per our experience, the advantage of sequential ultracentrifugation is the production of exosomes with high purity and cost-efficiency, however, it has disadvantage of low yield.
In order to allow the injected exosomes to cover most of the left ventricle and avoid leakage, we performed one intramyocardial injection using a 31 G insulin needle with the tip bent at about 20°. This technique is critical for the successful delivery of most exosomes into the myocardium and maximizes exposure of injected exosomes to host cardiomyocytes. In addition, we recommend injecting exosomes into the central region of the anterior wall of the left ventricle. After the injection is completed, we usually hold the needle at the injection site for 1 min to prevent liquid leakage. Successful injections were confirmed by the presence of a translucent edema around the injection site.
In our study, we found that allogeneic MPC-Exo transplantation can transiently restore dystrophin expression in heart and improve cardiac function in MDX mice22, which may provide new strategies for symptom relief in DMD patients. Since we observed recovered dystrophin protein expression in MDX mouse hearts, we assume that this is the mechanism for improved heart function, however, we cannot exclude other mechanisms, such as anti-inflammation; a recent report demonstrated that mesenchymal stem cell-derived exosomes improve the microenvironment of infarcted myocardium contributing to angiogenesis and anti-inflammation29, moreover, Aminzadeh et al.30 recently reported that cardiosphere-derived cells (CDCs) and their exosomes could transiently restore the expression of full length dystrophin in DMD mice. Considering that DMD is a systemic disease involving multiple organs, the local myocardial delivery of exosome is not suitable for treating respiratory failure due to diaphragm myopathy. Thus, systemic administration of exosomes, such as intravenous injection, has therapeutic potential, however, the major challenge is to develop an effective strategy for targeting exosomes to multiple muscle tissues. More importantly, exosome treatment has only a transient effect on partially restoring dystrophin expression, and improving heart function. More effective, longterm DMD treatment is needed.
Acknowledgments
Tang were partially supported by the American Heart Association: GRNT31430008, NIH-AR070029, NIH-HL086555, NIH-HL134354.
Footnotes
Disclosures
All authors declares that they have no conflicts of interest.
Video Link
The video component of this article can be found at https://www.jove.com/video/59320/
References
- 1.Yiu EM, Kornberg AJ Duchenne muscular dystrophy. Journal of Paediatrics and Child Health. 51 (8), 759–764, (2015). [DOI] [PubMed] [Google Scholar]
- 2.Nudel U, et al. Duchenne muscular dystrophy gene product is not identical in muscle and brain. Nature. 337 (6202), 76–78, (1989). [DOI] [PubMed] [Google Scholar]
- 3.Rae MG, O'Malley D Cognitive dysfunction in Duchenne muscular dystrophy: a possible role for neuromodulatory immune molecules. Journal of Neurophysiology. 116 (3), 1304–1315, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mah JK, et al. A systematic review and meta-analysis on the epidemiology of Duchenne and Becker muscular dystrophy. Neuromuscular Disorders. 24 (6), 482–491, (2014). [DOI] [PubMed] [Google Scholar]
- 5.D'Amario D, et al. A current approach to heart failure in Duchenne muscular dystrophy. Heart. 103 (22), 1770–1779, (2017). [DOI] [PubMed] [Google Scholar]
- 6.Koeks Z, et al. Clinical Outcomes in Duchenne Muscular Dystrophy: A Study of 5345 Patients from the TREAT-NMD DMD Global Database. Journal of Neuromuscular Diseases. 4 (4), 293–306, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kamdar F, Garry DJ Dystrophin-Deficient Cardiomyopathy. Journal of the American College of Cardiology. 67 (21), 2533–2546, (2016). [DOI] [PubMed] [Google Scholar]
- 8.Wang Z, et al. Regenerative Therapy for Cardiomyopathies. Journal of Cardiovascular Translational Research. (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fayssoil A, Nardi O, Orlikowski D, Annane D Cardiomyopathy in Duchenne muscular dystrophy: pathogenesis and therapeutics. Heart Failure Reviews. 15 (1), 103–107, (2010). [DOI] [PubMed] [Google Scholar]
- 10.Hagan M, Ashraf M, Kim IM, Weintraub NL, Tang Y Effective regeneration of dystrophic muscle using autologous iPSC-derived progenitors with CRISPR-Cas9 mediated precise correction. Medical Hypotheses. 110 97–100, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Quinlan JG, et al. Evolution of the mdx mouse cardiomyopathy: physiological and morphological findings. Neuromuscular Disorders. 14 (8-9), 491–496, (2004). [DOI] [PubMed] [Google Scholar]
- 12.Siemionow M et al. Creation of Dystrophin Expressing Chimeric Cells of Myoblast Origin as a Novel Stem Cell Based Therapy for Duchenne Muscular Dystrophy. Stem Cell Reviews and Reports. 14 (2), 189–199, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sienkiewicz D, Kulak W, Okurowska-Zawada B, Paszko-Patej G, Kawnik K Duchenne muscular dystrophy: current cell therapies. Therapeutic Advances in Neurological Disorders. 8 (4), 166–177, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Y, et al. Long-term engraftment of myogenic progenitors from adipose-derived stem cells and muscle regeneration in dystrophic mice. Human Molecular Genetics. 24 (21), 6029–6040, (2015). [DOI] [PubMed] [Google Scholar]
- 15.Ju C, et al. Transplantation of Cardiac Mesenchymal Stem Cell-Derived Exosomes for Angiogenesis. Journal of Cardiovascular Translational Research. 11 (5), 429–437, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ju C, et al. Transplantation of Cardiac Mesenchymal Stem Cell-Derived Exosomes Promotes Repair in Ischemic Myocardium. Journal of Cardiovascular Translational Research. 11 (5), 420–428, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruan XF, et al. Suxiao Jiuxin pill promotes exosome secretion from mouse cardiac mesenchymal stem cells in vitro. Acta Pharmacologica Sinica. 39 (4), 569–578, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ruan XF, et al. Exosomes from Suxiao Jiuxin pill-treated cardiac mesenchymal stem cells decrease H3K27 demethylase UTX expression in mouse cardiomyocytes in vitro. Acta Pharmacologica Sinica. 39 (4), 579–586, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen Y, Tang Y, Fan GC, Duan DD Extracellular vesicles as novel biomarkers and pharmaceutic targets of diseases. Acta Pharmacologica Sinica. 39 (4), 499–500, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen Y, Tang Y, Long W, Zhang C Stem Cell-Released Microvesicles and Exosomes as Novel Biomarkers and Treatments of Diseases. Stem Cells International. 2016 2417268, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murphy C, et al. Emerging role of extracellular vesicles in musculoskeletal diseases. Molecular Aspects of Medicine. 60 123–128, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Su X, et al. Exosome-Derived Dystrophin from Allograft Myogenic Progenitors Improves Cardiac Function in Duchenne Muscular Dystrophic Mice. Journal of Cardiovascular Translational Research. (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu G, et al. Exosome-mediated shuttling of microRNA-29 regulates HIV Tat and morphine-mediated neuronal dysfunction. Cell Death & Disease. 3 e381, (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bayoumi AS, et al. A carvedilol-responsive microRNA, miR-125b-5p protects the heart from acute myocardial infarction by repressing pro-apoptotic bak1 and klf13 in cardiomyocytes. Journal of Molecular and Cellular Cardiology. 114 72–82, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Y, et al. Exosomes/microvesicles from induced pluripotent stem cells deliver cardioprotective miRNAs and prevent cardiomyocyte apoptosis in the ischemic myocardium. International Journal of Cardiology. 192 61–69, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheruvanky A, et al. Rapid isolation of urinary exosomal biomarkers using a nanomembrane ultrafiltration concentrator. American Journal of Physiology-Renal Physiology. 292 (5), F1657–1661, (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oksvold MP, Neurauter A, Pedersen KW Magnetic bead-based isolation of exosomes. Methods in Molecular Biology. 1218 465–481, (2015). [DOI] [PubMed] [Google Scholar]
- 28.Pedersen KW, Kierulf B, Neurauter A Specific and Generic Isolation of Extracellular Vesicles with Magnetic Beads. Methods in Molecular Biology. 1660 65–87, (2017). [DOI] [PubMed] [Google Scholar]
- 29.Teng X, et al. Mesenchymal Stem Cell-Derived Exosomes Improve the Microenvironment of Infarcted Myocardium Contributing to Angiogenesis and Anti-Inflammation. Cellular Physiology and Biochemistry. 37 (6), 2415–2424, (2015). [DOI] [PubMed] [Google Scholar]
- 30.Aminzadeh MA, et al. Exosome-Mediated Benefits of Cell Therapy in Mouse and Human Models of Duchenne Muscular Dystrophy. Stem Cell Reports. 10 (3), 942–955, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]