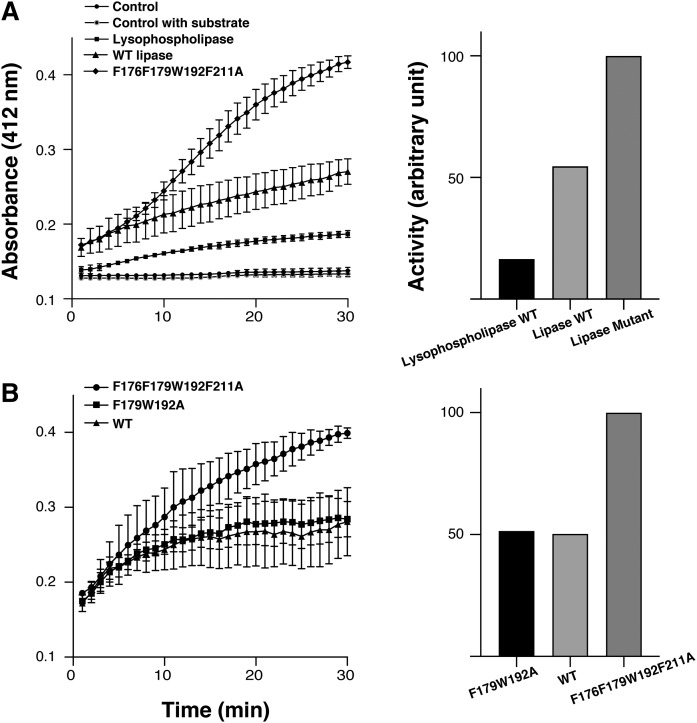
Fig. 4.
Lipase and lysophospholipase activity. A (Left): Activity of wild-type CAlipase (filled triangles ▲) and lysophospholipase (filled squares ■) was measured by colorimetry. The wild-type CAlipase hydrolyzes both substrates but preferred activity toward lipase substrate is observed. The active site shielding residues mutant F176F179W192F211A (filled diamonds ◆) indicates higher activity. A (Right): Relative initial rate of hydrolysis. Lipolytic activity is normalized to the active site shielding residue mutant. B: To compare the mutants’ activity, the lipase activity of active site shielding residue mutant F176F179W192F211A (filled circles ●) was compared with wild-type CAlipase (filled squares ■) and lipophilic path-forming residue mutant F179W192A (filled triangles ▲). The active site shielding residue mutant showed higher activity. Values are the mean of three separate determinations.