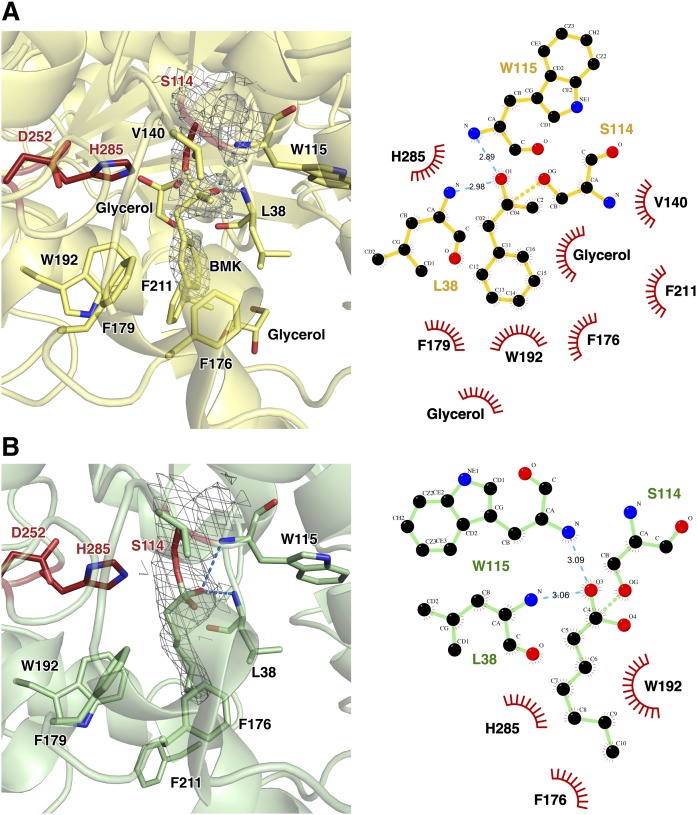
Fig. 7.
Modified serine at residue 114. A: The crystal structure of blocked Ser114 with BMK (blocked structure). The catalytic triad is shown as red sticks. Ligplot showing residues involved in interactions between BMK and CAlipase. The covalently bound BMK forms hydrogen bonds with Leu38 and Trp115. The methyl and benzene moieties are stabilized predominately by hydrophobic interactions with Val140, Phe176, Phe179, Trp192, and Phe211. B: Ser114 is modified as the fatty acid bound form in open structure by introduction of LPC. Ligplot analysis for bound LPC and CAlipase is shown. The hydrophobic interactions between Val140, Phe179, and Phe211 are absent due to the lack of methyl and benzyl groups.