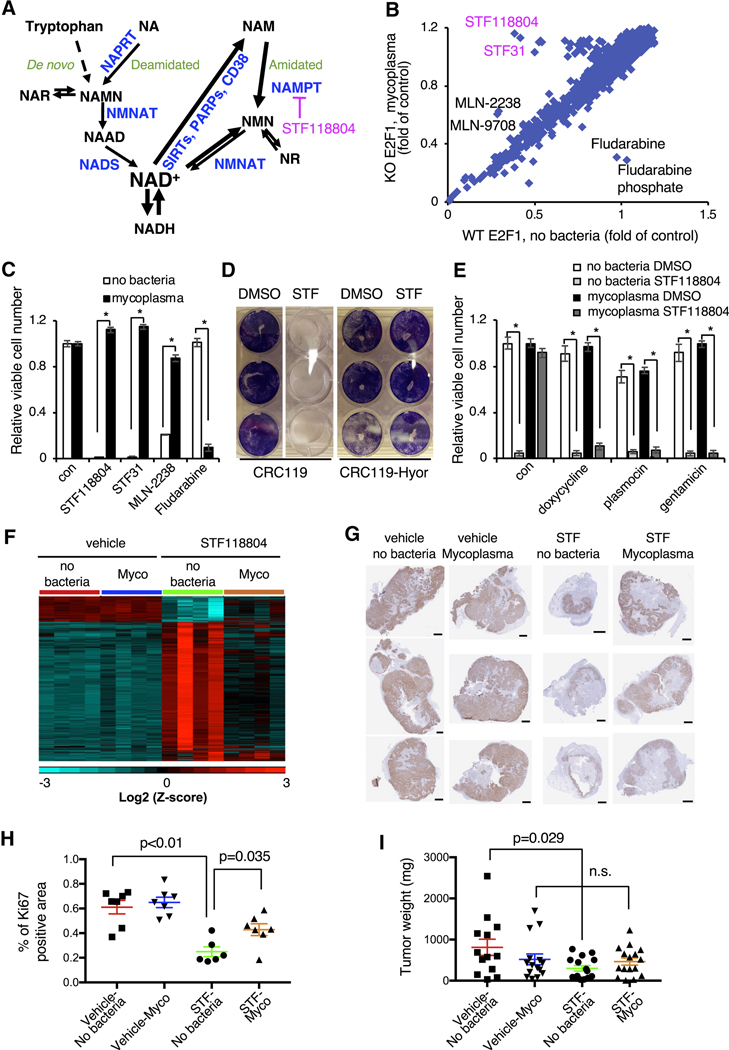
Figure 1. Mycoplasma infection confers human cells with resistance to NAMPT inhibitors.
(A) NAD biosynthesis pathway. NAR: nicotinic acid riboside; NAMN: nicotinic acid mononucleotide; NAAD: nicotinic acid adenine dinucleotide; NMN: nicotinamide mononucleotide; NR: nicotinamide riboside; NADS: NAD Synthetase; NAPRT: Nicotinic Acid Phosphorybosyltransferase; NMNAT: Nicotinamide Mononucleotide Adenylyltransferase.
(B) Drug screen in H1299 cells. WT and E2F1 KO H1299 cells were treated with 1 μM compounds from the bioactive compound library and viability was measured 48 hours later by CellTiter-Glo assay. E2F1 KO cells were subsequently found to be infected with mycoplasma.
(C) Mycoplasma infection confers resistance to NAMPT and proteasome inhibitors but sensitizes to fludarabine. CRC119 cells incubated with a supernatant from a mycoplasma-infected culture or a control medium, and treated with 100 nM STF118804, 1 μM STF31, 50 nM MLN2238 or 1μM fludarabine or control for 68 hours. Cell viability was measured by CellTiter-Glo assay (n=3, values are normalized to the corresponding DMSO controls and expressed as mean ±SD, *p<0.05).
(D) Mycoplasma hyorhinis confers mammalian cells with resistance to STF118804. CRC119 cells or CRC119-Hyor cells chronically infected with Mycoplasma hyorhinis were seeded in 12-well plates. Next day, cells were treated with 100 nM STF-118804. Seventy-two hours later cells were washed and adherent cells were stained with crystal violet.
(E) Elimination of mycoplasma sensitizes cells to NAMPTi-induced toxicity. Clean and mycoplasma-infected CRC119 cells were treated with 1 μg/ml doxycycline 25 μg/ml plasmocin, 400 μg/ml gentamicin or with medium control for 24 hours, then co-treated with antibiotics and 100 nM STF118804 or DMSO control for an additional 48 hours. Cell viability was measured by CellTiter-Glo assay (n=3, values are normalized to the corresponding DMSO controls and expressed as mean ±SD, *p<0.05).
(F) Mycoplasma infection of tumors attenuates the transcriptional response to STF118804 treatment. Clean (no bacteria) or M. hyorhinis-infected (Mycoplasma) HCT116 cells were xenografted into nude mice, then mice were treated with 15 mg/Kg STF118804 or vehicle control as described in STAR Methods. Tumor transcriptomes were profiled by RNA-seq. Heatmap represents the relative expression levels of 4183 genes differentially expressed between vehicle-and STF188804-treated clean tumors following hierarchical clustering of the genes (n=4, cut-offs: mean expression>20, two-fold change and p<0.05).
(G-I) Mycoplasma infected tumors are resistant to STF118804-induced inhibition of proliferation. Clean (no bacteria) or M. hyorhinis-infected (Myco) HCT116 cells were xenografted into nude mice. Eighteen days later, mice were treated with 30 mg/Kg STF118804 or vehicle control twice daily for nine days. Xenograft tumors were stained for a cell proliferation marker, Ki67 (G) and percentage of Ki67-positive area in each tumor section was calculated (H) (n=6–7, values are expressed as mean ±SEM). (I) Tumor weights (n=13–16, values are expressed as mean ±SEM,n. s., not significant). Bars in (G), 1 mm. See also Figures S1 and S2.