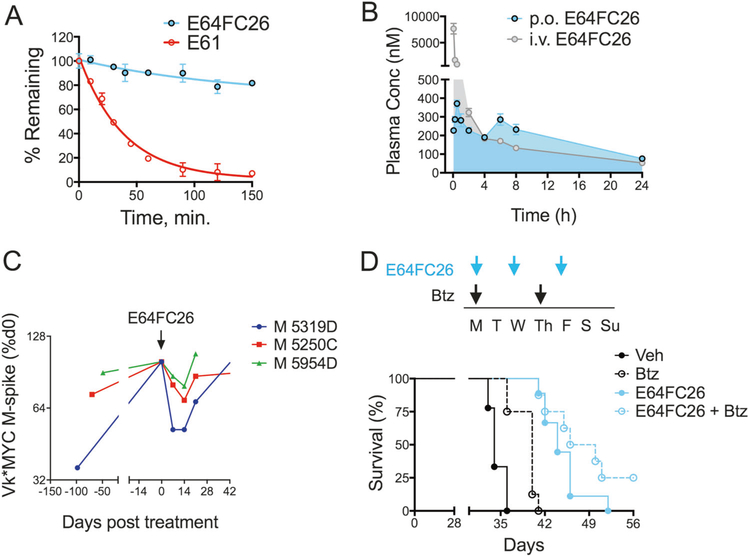
Fig. 6.
E64FC26 ADME-PK and in vivo anti-MM activity. a The stability of E64FC26 and E61 against oxidative metabolism by human liver microsomes. b PK of E64FC26 was measured in CD-1 mice. E64FC26 was administered i.v. (2 mg/kg; gray tracing) or p.o. (5 mg/kg; blue tracing) and plasma drug concentrations were measured over a 24 h period using an optimized LC-MS detection protocol. c Vk*MYC transgenic mice were treated with E64FC26 (2 mg/kg, i.p., 3 days/week) for two consecutive weeks. Blood serum M-protein levels were measured on days 0, 7, and 14 of treatment. M-spike data are shown as a percentage of day 0 for three individual mice. d NSG mice were xenotransplanted with MM.1S cells (1 × 106 cells, i.v.) and randomized to receive vehicle, Btz (0.25 mg/kg, i.p.), E64FC26 (2 mg/kg, i.p.), or combination treatment with both agents. The 7-day dosing schedule is shown (top panel). Treatment was initiated 7 days post-xenotransplantation. Kaplan–Meier survival curves (bottom panel) from NSG mice treated as described are shown. Statistical significance was determined by Wilcoxon rank-sum test (N = 10). Vehicle vs. Btz (P = 0.001), Vehicle vs. E64FC26 (p < 0.0001), Vehicle vs. E64FC26 + Btz (P < 0.0001)