Although not as ubiquitous as antibacterial susceptibility testing, antifungal susceptibility testing (AFST) is a tool of increasing importance in clinical microbiology laboratories. The goal of AFST is to reliably produce MIC values that may be used to guide patient therapy, inform epidemiological studies, and track rates of antifungal drug resistance. There are three methods that have been standardized by standards development organizations: broth dilution, disk diffusion, and azole agar screening for Aspergillus.
KEYWORDS: antifungal susceptibility testing, CLSI, EUCAST, epidemiological cutoff value, breakpoints, antifungal resistance
SUMMARY
Although not as ubiquitous as antibacterial susceptibility testing, antifungal susceptibility testing (AFST) is a tool of increasing importance in clinical microbiology laboratories. The goal of AFST is to reliably produce MIC values that may be used to guide patient therapy, inform epidemiological studies, and track rates of antifungal drug resistance. There are three methods that have been standardized by standards development organizations: broth dilution, disk diffusion, and azole agar screening for Aspergillus. Other commonly used methods include gradient diffusion and the use of rapid automated instruments. Novel methodologies for susceptibility testing are in development. It is important for laboratories to consider not only the method of testing but also the interpretation (or lack thereof) of in vitro data.
INTRODUCTION
The amount of antifungal susceptibility testing (AFST) being performed has increased in recent years for a myriad of reasons. The number of patients with risk factors for invasive fungal infection (IFI) (profound immunosuppression, exposure to long courses of broad-spectrum antibiotics, implanted medical devices) has increased in recent decades and with that has come the concomitant increase in IFIs. The echinocandin class of antifungals has been added, and the number of clinically available triazoles has increased, expanding the antifungal armamentarium. In addition, acquired resistance has emerged in fungal species such as Candida glabrata, Candida auris, and Aspergillus fumigatus. With these changes in the patient population and the availability of choice in antifungal therapy, the need for accurate in vitro susceptibility data is greater than ever.
AFST is often performed by clinical microbiology laboratories as a tool to aid in the selection of the optimal antifungal agent. By definition, it provides an in vitro measure of susceptibility and resistance by determining the concentration of drug required to inhibit an organism to a specified degree, termed the MIC. Ideally, a reliable prediction can then be made as to patient outcome relative to therapy. The demand for in vitro susceptibility testing for antifungal drugs did not exist a few decades ago, when similar testing was already performed for antibacterial drugs. The first antifungal agent, amphotericin B (AmpB), was introduced in the 1950s, nearly 30 years after the discovery of the first antibacterial agents. For a long time, AmpB was the only therapeutic option for fungal infections, and as such, the necessity for testing its activity against clinical isolates did not exist. The decades following would see the introduction of less toxic antifungals such as 5-flucytosine, the imidazole and triazole antifungal classes, and finally the echinocandins. As more therapeutic choices became available, the value of detecting antifungal resistance, both intrinsic and acquired, and the need for optimization of the antifungal choice increased.
With the increasing need to predict which fungal infections would or would not respond to treatment came the necessity of standardized testing methods for the laboratory. Reproducible techniques for antifungal susceptibility testing are crucial to reliably predict outcome, to create uniformity in reporting, and to facilitate interlaboratory comparisons and agreement. Many factors influence the outcome of in vitro susceptibility testing, including endpoint definition, inoculum size of the organism, time of incubation, temperature of incubation, and medium used for testing (1). Minimizing the influence of variability of such factors on the final MIC value was the key rationale behind standardization. Without standardization, initial interlaboratory comparisons of MIC values were less than optimal (2, 3).
The Clinical and Laboratory Standards Institute (CLSI), formerly the National Committee for Clinical Laboratory Standards (NCCLS), formed in 1968 with the goal of harmonizing quality control (QC) and standardization of susceptibility testing for all pathogens. The first report on the state of laboratory testing of antifungal susceptibility in the United States, M20-CR, was completed in 1985 and concluded that intralaboratory agreement for antifungal susceptibility testing was unacceptable. Following this report, the antifungal subcommittee began the process of standardization of susceptibility testing, culminating with the creation of document M-27 (4).
Like the CLSI, the European Committee on Antimicrobial Susceptibility testing (EUCAST) was formed to standardize technical aspects of in vitro antimicrobial susceptibility testing and to develop breakpoints. Originally formed jointly by the European Society of Clinical Microbiology and Infectious Diseases (ESCMID), the European Centers for Disease Control and Prevention (ECDC), and European national breakpoint committees, EUCAST harmonized standards for countries both within and outside Europe. In 1997, the EUCAST antifungal susceptibility testing subcommittee formed, and their first published standard of susceptibility testing for both yeasts and molds was released in 2008 (5).
ROLE OF ANTIFUNGAL SUSCEPTIBILITY TESTING IN THE CLINICAL LABORATORY
In most clinical microbiology laboratories where AFST is available, it is performed primarily for yeasts, although susceptibility testing for molds, performed with less frequency in routine clinical laboratory work, is sometimes available. But susceptibility testing is not advised for every fungal pathogen that is detected in culture. Isolates from nonsterile body sites are often not treated and may not require AFST. The antifungal drug of choice for some pathogens can be empirically assumed by the proper identification of the pathogen and, barring clinical failure, may not require susceptibility testing. An example would be Aspergillus fumigatus in the United States, where voriconazole is used empirically and resistance has not been widely identified. Additionally, for fungal pathogens with no established interpretive criteria (either a breakpoint or an epidemiological cutoff value), an MIC value may be obtained but may have no clear clinical interpretation.
The most value in AFST is gained when the fungal infection is invasive, when acquired drug resistance is suspected, or when the patient is unexpectedly failing therapy. For each of these scenarios, knowing the in vitro susceptibility pattern would inform the clinician when making therapeutic choices or changes. These indications for routine testing have been defined for some species of Candida only (6, 7); the validity and potential benefit of routine AFST for other clinically significant fungal pathogens have not yet been widely characterized, although there are some recommendations for susceptibility testing of Aspergillus, following the establishment of breakpoints for voriconazole against A. fumigatus by the CLSI (8, 9) and for amphotericin B, isavuconazole, itraconazole, posaconazole, and voriconazole against a number of Aspergillus species by EUCAST (10–12).
Antifungal susceptibility testing can be beneficial beyond the selection of an antifungal agent for individual patient therapy. The rates of drug resistance that are obtained from AFST data are valuable on both a small scale (institutional) and large scale (regional/international/continental). Institutionally, rates of antifungal resistance are an important part of epidemiological studies used to determine susceptibility profiles and empirical therapy for “local” fungal pathogens. This can be used to establish antibiograms for a health care center or hospital and, in turn, set institutional antifungal stewardship. On a larger scale, the surveillance and monitoring of rates of antifungal drug resistance are important components of developing robust data sets that will ultimately be used to define or refine breakpoints or epidemiological cutoff values. This is discussed further below.
AFST is also valuable in the development of novel antifungal agents. The establishment of quality control (QC) organisms and QC ranges for those organisms is essential for the eventual standardization of testing (13). AFST can then be used to determine the degree of activity of a new potential antifungal against a panel of isolates of various species across a number of laboratories, with the QC serving to ensure that the testing is standardized and the results are comparable.
The changing epidemiology of fungal infections has also been paramount in the changing role of AFST in clinical laboratories. Although many aspects of Candida epidemiology have remained stable over decades, a trend toward non-albicans species of Candida has occurred, and with that an increase in antifungal resistance (14–17). The most common agent of aspergillosis, Aspergillus fumigatus, has shown increasing levels of azole resistance in many parts of the world, especially Europe (18–20). Moreover, the emergence of Candida auris, for which antifungal resistance is the norm rather than the exception, has challenged the traditional paradigms of Candida pathogens for laboratorians and clinicians (21–23). These and other emerging fungal pathogens will require frequent susceptibility testing as a part of their management, supporting the need for reliable, cost-effective, and easy to perform testing strategies.
REFERENCE METHODS FOR SUSCEPTIBILITY TESTING
Broth Dilution for Yeasts
Broth dilution is the process by which the in vitro activity of a drug is measured against a test organism in liquid culture with a known concentration of drug. As one of the first methodologies developed (24, 25), it has historically been the most commonly used technique for antifungal susceptibility testing in the United States. Generally speaking, a test tube or microtiter plate which contains a standard medium, 2-fold serial dilutions of an antifungal drug, and a predetermined amount of test organism inoculum is used. The endpoint of this assay is read as the concentration of drug that decreases growth of the test organism to a prespecified degree. While macrodilution was the original method pursued, microdilution has been adopted more readily for mainstream use in the clinical laboratory.
There are two standards which are accepted for clinical laboratory broth microdilution testing: those established by the CLSI and those established by EUCAST (26, 27). Both organizations use the same medium, albeit with different glucose concentrations, and use the same criteria to define the endpoint of the assay. They also currently use similar criteria to develop clinical breakpoints and therefore interpretations for antifungal resistance and/or susceptibility, which ultimately produce similar results. But these standards have important differences, the specifics of which are discussed below and summarized in Table 1.
TABLE 1.
Comparison of key differences between CLSI and EUCAST methods of broth microdilution
| Parameter | CLSI M27-A4 | EUCAST E.DEF 7.3.1 |
|---|---|---|
| Glucose content of RPMI medium | 0.2% glucose | 2% glucose |
| Preparation of antifungal agent | Prepare stock concentration of at least 1,280 μg/ml or 10 times the highest concentration to be tested, whichever is greater | Prepare at concentrations at least 200 times higher than the highest concentration to be tested in the plate |
| Preparation of organism | Subculture yeast at least twice | The number of subcultures is not defined |
| Microdilution plate | Plates with U-shaped wells | Tissue-treated plates with flat-bottomed wells |
| Inoculum size | Yeasts, 0.5 × 103 to 2.5 × 103 cells/ml; filamentous fungi (nondermatophyte), 0.4 × 104 to 5 × 104 cells/ml; filamentous fungi (dermatophyte), 1 × 103 to 3 × 103 cells/ml | Yeasts, 1 × 105 to 5 × 105 cells/ml; filamentous fungi, 2 × 105 to 5 × 105 cells/ml |
| Reading method | Visual | Spectrophotometric |
| Cryptococcus spp. | Read at 72 h of incubation | Read at 48 h of incubation |
| Amphotericin B reading | 100% decrease in growth, or the first optically clear well | ≥90% decrease in growth |
| Miscellaneous | Recommends against the use of low-evaporation lids |
Broth Microdilution for Yeasts Using the CLSI Standard
In 1992, the CLSI Subcommittee on Antifungal Susceptibility Tests published its first standard for the reproducible susceptibility testing of yeasts, M27-P (28). This document outlined the provisional ranges of MIC and breakpoints for several antifungal drugs and their action against yeasts. Over the course of several revised standards, this document evolved to the current iteration, the 4th edition of M27 (M27-Ed4), and currently covers antifungal agent selection and preparation, test procedure, and QC requirements (27). M27-Ed4 describes methods for testing Candida and Cryptococcus species, but the methods can be applied broadly to any yeast that will grow in the standard RPMI medium; this includes most yeast species, with the notable exception of lipophilic Malassezia species, which require supplemental lipids. A separate CLSI document, M60, details the most current performance standards for the antifungal susceptibility testing of yeasts and contains MIC breakpoints, interpretive categories, and MIC ranges for QC organisms (29).
The CLSI guidelines for broth microdilution as described in the M27 document require 96-well microdilution plates of untreated polystyrene with U-shaped wells for all testing. The medium is RPMI 1640 culture medium (with l-glutamine and phenol red as a pH indicator but without bicarbonate), buffered to a pH of 7 with 3-(N-morpholino)propanesulfonic acid (MOPS), as this has been shown to produce consistent results and was used to develop the standard. The RPMI 1640 medium should have a glucose concentration of 0.2%.
For preparation of the antifungal agent, the CLSI suggests acquisition of drug powder from manufacturers or other commercial sources as opposed to pharmacy stock. Pharmacy stock should not be used due to the presence of excipients in the powder that may interfere with testing. When determining the amount of drug powder needed for solution, either of the following two formulas is recommended:
The powder should be weighed on a calibrated analytical balance. The log2 concentration range of the antifungal drug should be selected to encompass the QC range described in the M60 document and then extend high enough to identify any resistant isolates. Solvents other than water may be used when appropriate and include analytical-grade dimethyl sulfoxide (DMSO), ethyl alcohol, polyethylene glycol, and carboxymethyl cellulose, although DMSO is recommended for the all of the currently licensed antifungals listed in M60 (27).
For preparation of the inoculum, the CLSI recommends that all organisms be subcultured at least twice at 35°C using antimicrobial-free medium, such as Sabouraud’s dextrose agar or potato dextrose agar, to ensure purity and organism viability. Approximately five colonies at least 1 mm in diameter should be picked and suspended in sterile saline or water, vortexed, and adjusted using a spectrophotometer to a transmittance that equals a 0.5 McFarland standard at a wavelength of 530 nm. This stock solution is used to make a working solution by preparing a 1:100 dilution, followed by a 1:20 dilution, with RPMI 1640 culture medium. The final resulting inoculum will be between 0.5 × 103 and 2.5 × 103 cells per ml.
After drug plates are inoculated, they are incubated without agitation at 35°C ± 2°C for 24 h before reading. The exception is Cryptococcus species isolates, which should be held for 72 h prior to reading. The growth control well is inspected for the presence or absence of growth. Plates for which there is insufficient growth in the control well to make an accurate reading may be held for a further 24 h. All plates are then read visually under normal laboratory lighting using a mirror viewer. Wells should be scored for growth compared to that of the drug-free control well. For azoles, echinocandins, and flucytosine, the MIC is set as the lowest drug concentration at which there is at least a 50% decrease in growth compared to that of the drug-free well, and the MIC for amphotericin B is the concentration that produces a 100% decrease in growth, or the first optically clear well.
Because the final MIC is determined by visual inspection at 50% growth inhibition, the MIC value that is determined using CLSI methodology may be somewhat subjective. In addition, the range of the acceptable inoculation size, the fluctuation in incubator temperatures, and the exact time of reading may lead to intralaboratory variation when reading the same isolate with the same antifungal. This is why QC standards are given as a range and why a difference of 2 log2 dilutions is determined to be essential agreement (EA) for antifungal MIC values determined at different times or in different laboratories (13, 30).
Broth Microdilution for Yeasts Using the EUCAST Standard
In contrast to the CLSI, EUCAST document E.DEF 7.3.1 recommends the use of flat-bottomed microdilution plates for testing and also specifies that low-evaporation lids should not be used, as this might interfere with oxygen concentrations (26). This document goes on to recommend the use of tissue-treated microdilution plates specifically, as preliminary unpublished data suggest that tissue-treated and non-tissue-treated plates produce different MIC values.
Like the CLSI, EUCAST recommends that completely synthetic growth medium be used for antifungal susceptibility testing and mandates the use of RPMI 1640 medium supplemented with glucose and MOPS buffered to a pH of 7. However, unlike the CLSI, EUCAST recommends a 2% glucose concentration to facilitate optimal organism growth and determination of MIC endpoints.
Recommendations for the preparation of antifungal agents as listed in the EUCAST document (selection and weighing of drug powders, preparation of stock solutions, preparation of working solutions, selection of drug concentration ranges) are identical to those defined by CLSI document M27 with a single exception. For preparation of a stock solution, EUCAST recommends using concentrations at least 200 times higher than the highest concentration to be tested, which contrasts with the CLSI recommendation of preparing stock concentrations of at least 1,280 μg/ml or 10 times the highest concentration to be tested, whichever is greater.
The EUCAST recommendation for inoculum preparation is like that of the CLSI. All organisms should be subcultured onto nonselective medium such as Sabouraud’s dextrose agar or potato dextrose agar at 34 to 37°C, although the number of subcultures is not defined. Approximately five colonies at least 1 mm in diameter should be picked and suspended in sterile distilled water and adjusted using a spectrophotometer to a transmittance that equals a 0.5 McFarland standard at a wavelength of 530 nm. This stock solution is then used to make a working solution by preparing a 1:10 dilution in sterile distilled water. The final resulting inoculum size, which is greater than the CLSI final inoculum size, will be between 1 × 105 and 5 × 105 cells per ml.
After drug plates are inoculated, they are incubated without agitation at 35 ± 2°C for 24 ± 2 h before reading (plates containing Cryptococcus species isolates are to be held for 48 h prior to reading per EUCAST guidelines). Plates for which the growth control has not reached an absorbance of ≥0.2 at a wavelength of 530 nm are incubated for an additional 12 to 24 h. All plates are then read using a microdilution plate reader at a recommended wavelength of 530 nm (although other wavelengths, such as 405 nm or 450 nm, can be used) with subtraction of the blank or background from the reading of each well. For azoles, echinocandins, and flucytosine, the MIC is set as the lowest drug concentration at which there is a ≥50% decrease in growth, and for amphotericin B, the MIC is set as the lowest drug concentration at which there is a ≥90% decrease in growth.
It should be noted that although the CLSI has developed breakpoints for caspofungin against several species of yeast, problems have been noted with testing of caspofungin (31, 32). High interlaboratory variability, with some laboratories having an inordinate number of susceptible isolates classified as resistant, has led to the recommendation that caspofungin alone should not be used as a surrogate for susceptibility to echinocandins. For this reason, EUCAST has not defined yeast breakpoints for caspofungin. These problems are not encountered when testing with anidulafungin or micafungin, which can be used as surrogates for caspofungin resistance (33, 34).
Broth Microdilution for Molds
Both the CLSI and EUCAST have broth microdilution standards for mold AFST (35, 36). These protocols are like the protocols for yeast testing with some notable changes in each protocol, which are discussed separately.
The filamentous fungus protocol from the CLSI uses the same plates that are used for the yeast testing. However, the inoculum used for filamentous fungi is different. While the yeast protocol uses 0.5 × 103 to 2.5 × 103 cells per ml, the filamentous fungus protocol uses 0.4 × 104 to 5 × 104 conidia per ml for nondermatophytes and 1 × 103 to 3 × 103 conidia for dermatophytes. When the density of the conidia is measured using a spectrophotometer, the correct absorbance will depend on the species, because of the vastly different sizes of the conidia of filamentous fungi (35). While most yeast species are incubated for 24 h prior to reading the plates, filamentous fungi are incubated for 46 to 50 h, the exceptions being Mucorales, which are incubated for 24 h, and dermatophytes, which are incubated for up to 96 h (4 days).
Although broth microdilution plates for yeast species and filamentous fungi are both read visually using CLSI standards, the similarities end there. The azoles and amphotericin B both prevent the germination of the mold conidia used for inoculation and are measured as a lack of growth. For this reason, the MIC for azoles and amphotericin B is the first well with 100% growth inhibition. This removes some of the interpretation that is seen with yeast species, as the endpoint is no growth rather than a 50% decrease, which can be interpreted differently by two technicians reading the same plate. However, for the echinocandins, the conidia do germinate, but at susceptible concentrations, growth is halted at the tip of the emerging hyphae. The result is a change in the pattern of growth, and this is measured as the minimal effective concentration (MEC), defined as the lowest concentration that leads to the growth of small, round, compact hyphal forms compared to the hyphal growth seen in the control well. This visible change in growth is due to the fact that the echinocandins are fungistatic against molds rather than fungicidal.
EUCAST broth microdilution for filamentous fungi also differs from the EUCAST yeast protocol. Like the CLSI, EUCAST uses the same plates for susceptibility testing of filamentous fungi and for yeast species. But unlike the CLSI, EUCAST uses a similar inoculum size, 2 × 105 to 5 × 105 CFU per ml, for molds and for yeast species. Incubation time is species dependent, as for the CLSI method, and the endpoint for azoles and amphotericin B is read as complete growth inhibition while that for the echinocandins is read as the MEC. An additional recommendation of EUCAST is that the plates be read visually with a black strip of paper covering half of the well. The sharpness of the paper as seen through the inoculum helps to determine whether there is any visible growth.
A comparison of the CLSI and EUCAST methods of broth dilution for yeasts and molds is shown in Table 1.
Disk Diffusion
The disk diffusion testing method is one of the oldest approaches to susceptibility testing and remains widely used in many clinical laboratories, especially in resource-limited settings (37). Disk diffusion testing is inexpensive, reproducible, and easy to interpret (38–40).
Disk diffusion testing involves the use of commercially prepared paper disks containing a fixed concentration of antifungal drug. The diameter of clearing produced around the disk, termed the zone of inhibition, relates to the diffusion rate of drug through the agar medium and to the susceptibility of the isolate to that drug. When zone diameters are correlated with MICs for a given fungus-antifungal combination, they may be translated into interpretive categories.
The CLSI has developed a standardized protocol for yeast susceptibility testing (41). The recommended medium for disk diffusion testing is Mueller-Hinton agar with 2% glucose and 0.5 μg/ml methylene blue dye (GMB) for maximum reproducibility of testing. The addition of glucose supports fungal growth, and the addition of methylene blue dye enhances the zone edge definition. The pH of the agar should be between 7.2 and 7.4 at room temperature. The CLSI recommends the same procedure for the preparation of inoculum for disk diffusion as for broth microdilution, with a final stock suspension of 1 × 106 to 5 × 106 cells per ml. This cell count produces confluent growth on the agar plate with most Candida species. A sterile cotton swab is dipped into the adjusted cell suspension, pressed firmly against the inside wall of the tube to remove excess liquid, and then used to streak the entire agar surface a total of 3 times, with rotation of the plate approximately 60° each time to ensure that the inoculum is evenly distributed across the plate. After the plate has been dried for at least 3 min but no more than 15 min, the disk(s) may be applied to the inoculated agar and pressed gently to ensure complete contact with the surface. If multiple disks are used, they should be no closer than 24 mm from center to center. Following 20 to 24 h of incubation at 35°C ± 2°C, the zone diameter for each disk is measured to the nearest millimeter at the point in which there is a prominent reduction of growth. CLSI document M60 provides zone diameter interpretive criteria for caspofungin, fluconazole, and voriconazole (29, 42).
The limitation of this methodology is that it does not provide an MIC value. Zone diameter interpretive criteria were developed by comparing the zone diameters to the MIC values of many isolates. Those values were then converted to interpretive criteria based on the overall correlation to MIC and patient outcome. What this means is that disk diffusion criteria can be developed only for fungus/antifungal combinations for which there are already breakpoints. Although this means that disk diffusion is useful for the >90% of Candida isolates that fall within the five species for which there are breakpoints, this method is not useful for emerging species or for newly developed antifungals because there is no way to interpret the result.
There have been some initial studies to look at the usefulness of disk diffusion for filamentous fungi (43–45). The correlation to broth microdilution is not very good, and there are no interpretive criteria by which to evaluate the results, but the CLSI has developed a standard for testing (46). Disk diffusion interpretive criteria for filamentous fungi are not forthcoming.
Disk diffusion is an ideal testing method for Candida global surveillance programs, as it is technically simple, inexpensive, and requires little equipment (47–49). Importantly, very good categorical agreement between disk diffusion results and MIC results obtained by broth microdilution have been established (38, 50–54). Categorical agreement between broth microdilution and disk diffusion tends to be >90% and in most cases >95%. In a very large study of 1,586 Candida species isolates and fluconazole, there was 93% categorical agreement, with only 0.3% major errors and 0.1% very major errors. For voriconazole, the categorical agreement reached 99% (49).
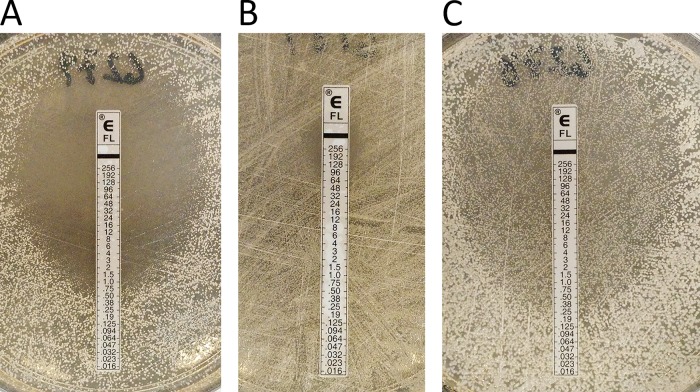
Agar Screening for Aspergillus Resistance
EUCAST has recently developed a standard for the determination of azole resistance in Aspergillus isolates using a four-well agar screening plate (E.DEF 10.1) (55–57). The assay consists of an RPMI 1640 agar plate with wells containing itraconazole (4 μg/ml), voriconazole (2 μg/ml), and posaconazole (0.5 μg/ml) and a drug-free well. Aspergillus conidia are inoculated on the plate, and growth is monitored for 48 h. Susceptible isolates can be reported as such, but growth in any well besides the growth control is an indication that resistance may be present and broth microdilution susceptibility testing should be performed.
COMMERCIAL TESTING METHODS
In addition to the reference methods described above, there are commercially available products for antifungal susceptibility testing. Their key differences are summarized in Table 2.
TABLE 2.
Widely used methodologies for antifungal susceptibility testing
| Methodology or system | Strengths | Weaknesses |
|---|---|---|
| Broth microdilution | Gold standard Can be used for both yeasts and filamentous fungi Methodologies are standardized and available for both yeasts and filamentous fungi Plates can be produced in the laboratory | Subjective interpretation by CLSI methodology, alleviated using EUCAST (spectrophotometer) |
| Labor-intensive to make plates and expensive to buy them commercially prepared | ||
| Technical training requirement is high | ||
| Can be used for any new antifungal that is discovered | ||
| Disk diffusion | A methodology has been standardized Inexpensive Disks can be produced in the laboratory | Very few interpretive criteria are available |
| Disk diameters are categorical only and do not directly correlate with MIC values | ||
| Good for resource-limited settings | Commercially available for only a few antifungals | |
| Gradient diffusion | Inexpensive compared to broth microdilution | Interpretation is somewhat subjective |
| Can be used for both yeasts and filamentous fungi | Must be purchased commercially | |
| Good for resource-limited settings | Not available for antifungals in development | |
| Provides an MIC value | ||
| Improved discrimination between amphotericin B-susceptible and -resistant isolates | ||
| Improved discrimination between true resistance and trailing growth phenomenon | ||
| YeastOne | Interpretation is less subjective than standard broth microdilution | Interpretation of trailing growth in azoles can be problematic Cannot be used for filamentous fungi Must be purchased commercially Even though it is broth microdilution, it does not follow CLSI or EUCAST guidelines for interpretation |
| Easier to train new technicians than standard broth microdilution | ||
| Long shelf life of dried trays | ||
| Does not require freezer storage | ||
| VITEK 2 | No subjectivity to interpretation | Expensive for both initial startup costs and for cartridges |
| Very easy to set up and perform | Cannot be used for filamentous fungi | |
| Dual use for laboratories that already use VITEK 2 for identification | Available for only a limited number of antifungals Overcalls resistance for some species (like Candida auris) | |
| Agar screening for Aspergillus | Easy to perform | It is only a screening test |
| Easy to interpret | Needs to be backed up with broth microdilution | |
| Inexpensive |
Gradient Diffusion Strips
An alternative to broth microdilution and disk diffusion is the use of gradient diffusion strips. These are thin strips of plastic or nitrocellulose containing a predefined, dried gradient of antifungal drug on one side and marked with a concentration scale on the other. After placement on a confluent lawn of fungal cells or conidia, gradient diffusion strips are incubated for 24 to 48 h, during which time the antifungal diffuses into the agar. The test is read using the zone of inhibition to mark the point at which the ellipse-shaped growth of fungi intersects with the strip, indicating an MIC value (Fig. 1A). Strips are available for all approved antifungal drugs, including the newest triazole drug, isavuconazole (bioMérieux, Hazelwood, MO; Liofilchem, Waltham, MA).
FIG 1.
Fluconazole gradient diffusion strips distinguish between isolates that are fluconazole susceptible (A), fluconazole resistant (B), or fluconazole susceptible but displaying heavy “trailing” growth (C).
In addition to its ease of use, an advantage that gradient diffusion strips have over disk diffusion is the ability to quantify antifungal susceptibility by generating an MIC value. The MIC values produced via gradient diffusion compare favorably to values generated by broth microdilution, with good essential agreement (EA) between the two methods in most cases (38, 45, 58–64). For 93 Candida species isolates and fluconazole, the EA between the Etest and EUCAST broth microdilution was 90% (65). Overall categorical agreement was not given, but there were no very major errors. For 133 Candida spp. tested against the echinocandins, EA ranged from 90% for caspofungin to 93% for anidulafungin (66). It should be noted here that the same problems that were outlined above for broth microdilution testing of caspofungin and yeasts may exist as well for gradient strip testing (31). In addition, even though it is known to be wild type for caspofungin, the QC MIC range for Candida krusei ATCC 6258 bisects the susceptible and resistant ranges when Etests are used (Etest package insert; bioMérieux, Marcy-l’Etoile, France; https://techlib.biomerieux.com/wcm/techlib/techlib/documents/docLink/Package_Insert/35904001-35905000/Package_Insert_-_9305056_-_D_-_en_-_Etest_-_AFST_WW.pdf).
Unlike disk diffusion, there is good evidence that gradient diffusion strips can be used for susceptibility testing of molds against the triazoles and amphotericin B, with the exception being the Mucormycetes and dermatophytes (44, 67–74). Against 24 isolates of A. fumigatus, including those with known resistance mechanisms, EA with broth microdilution ranged from 83 to 100% with no very major errors for gradient diffusion strips from two different manufacturers. Categorical agreement using the EUCAST breakpoints was 96 to 100% for itraconazole, voriconazole, and isavuconazole but only 33% and 83% for the two different manufacturers for posaconazole, with a high percentage of major errors due to overcalling resistance (67). For a set of 20 Fusarium species, the results were similar, with 95 to 100% EA with broth microdilution for amphotericin B, voriconazole, and posaconazole (68). In a very large study of 376 Aspergillus species isolates, EA with broth microdilution was 98% for voriconazole and 96% for itraconazole (69). In contrast, against 46 dermatophytes, the EA between gradient diffusion and broth microdilution was 20% for itraconazole and 52% for voriconazole (44).
A noteworthy advantage of antifungal testing with gradient diffusion strips versus broth microdilution is for the testing of amphotericin B. Broth microdilution testing of Candida spp. against amphotericin B reveals MIC values which are tightly clustered within a range of 0.25 to 1 μg/ml. However, when testing is performed using gradient diffusion strips, a much wider and diverse range of MIC values is obtained and discrimination between amphotericin B-susceptible and -resistant isolates may be accomplished for both Candida and Cryptococcus (75–79). This suggests that the testing of amphotericin B is more reliable when performed using gradient diffusion strips. However, the paucity of resistant isolates of most species and the lack of a molecular target for resistance testing leave the question of superiority still up for debate.
An additional value of gradient diffusion strips is the ability to distinguish between the trailing growth of some susceptible organisms from true microbiological resistance when broth microdilution is used as defined by the CLSI procedure. As discussed below, trailing growth is the result of residual organism growth beyond the MIC and in some circumstances can make the visualization of the MIC difficult and/or impossible. When a resistant isolate is tested using a gradient diffusion strip, the growth is confluent throughout the lawn of cells except for the ellipse (Fig. 1B). However, when a gradient diffusion strip is used for a susceptible isolate that exhibits trailing growth, there is an ellipse filled with microcolonies and the ellipse can be used to determine the intercept point with the gradient strip (Fig. 1C). In this way, the gradient diffusion strips can distinguish between isolates that are susceptible, resistant, or susceptible “trailing” and serve as another level of confirmation of MIC values for very heavily trailing isolates.
Sensititre YeastOne Assay
Sensititre YeastOne colorimetric antifungal panel (ThermoFisher Scientific, Waltham, MA [formerly TREK Diagnostic Systems]) is a commercially prepared broth microdilution plate. Like other forms of broth microdilution, the YeastOne panel consists of a 96-well plate containing serial dilutions of antifungal drug in defined medium. But, in addition, the wells contain the colorimetric growth indicator resazurin (alamarBlue), so fungal growth and MIC determination are based on a color change in the well rather than on a defined amount of growth. The plates are shipped dry, have a shelf life of approximately 24 months at room temperature, and are packaged individually, ideal for use in a clinical laboratory in which testing is performed infrequently.
Multiple studies have demonstrated high interlaboratory reproducibility using these panels, with reproducibility for essential agreement (EA) ranging from 90 to 99% (80–84). Additionally, the EA between Sensititre YeastOne and reference broth microdilution methods is high (60, 80, 84). Alexander et al. (60) found ≥92% EA for MICs for itraconazole, flucytosine, amphotericin B, and caspofungin, 82% EA for fluconazole, and 85% EA for voriconazole. Interpretive categorical agreement was 88% for this assay and was the lowest for C. glabrata and Candida tropicalis (60). Some of the comparative results that are not perfect are a result of the fact that the comparisons took place prior to changes in the broth microdilution standard. The EA was sometimes determined using YeastOne results read at 24 h and broth microdilution results read at 48 h. In addition, the categorical agreement was calculated using the old CLSI non-species-specific breakpoints. In a study that looked at 404 Candida isolates using the M27-A3 standard and M27-S4 breakpoints, 100% EA between reference methods and Sensititre YeastOne was observed. The categorical agreement ranged from 93.6% with caspofungin to 99.6% for micafungin. There was <1% very major or major errors combined (85).
While Sensititre YeastOne is neither designed nor recommended for use with filamentous fungi, there are several studies which indicate that it could be useful (86–92). Using 63 Aspergillus species isolates, EA with the CLSI M38-A standard was 93% for amphotericin B, 90% for itraconazole, and 83% for voriconazole, although agreement for some individual species was higher (86, 87). In similar studies, one group found 100% EA for Aspergillus species against voriconazole and 90% agreement against amphotericin B, while another group that tested 279 Aspergillus species isolates found 98% EA for both itraconazole and voriconazole but only 31% EA for amphotericin B (88, 91). In a comparative study of both Aspergillus and non-Aspergillus filamentous fungi of 18 different species against posaconazole, 97% EA with CLSI broth microdilution was achieved (89). Further investigation into the usefulness of this assay for filamentous fungi, including the establishment of species recommendations and quality control isolate ranges, is warranted.
Vitek 2 Yeast Panels
The Vitek 2 yeast susceptibility panel (bioMérieux, Hazelwood, MO, USA) is an automated approach to AFST that uses spectrophotometric readings to determine an MIC value for clinically relevant Candida species. The cards contain wells with dried concentrations of antifungal drugs in medium. After the isolate is adjusted to a standardized concentration in saline, it is used to rehydrate the drug wells and the card is placed into a Vitek 2 card reader/incubator. The growth within each well is monitored up to 36 h (an average of 12 to 14 h) by an optical scanner, and a report containing an MIC value along with the interpretive category for each antifungal on the card is generated.
The automation afforded by this assay is ideal for clinical microbiology laboratories with a high testing volume. It does not require specialized mycology training and is a rapid method of AFST that produces accurate results comparable to those of broth microdilution (93–103). EA with both CLSI and EUCAST reference methods is approximately >95% overall (97). A large multilaboratory study reported EA values for amphotericin B, flucytosine, and voriconazole of 99.1%, 99.1%, and 96.7%, respectively (93). EA values for caspofungin, micafungin, and posaconazole have been reported as 99.5%, 98.6%, and 95.6%, respectively (99). However, manual readings are not possible, and in the United States, only fluconazole, voriconazole, and caspofungin are approved by the Food and Drug Administration (FDA) for AFST via this system.
MISCELLANEOUS METHODS
Flow Cytometry
Flow cytometry, or fluorescence-activated cell sorting (FACS), is an existing technology that has been evaluated for use in antifungal susceptibility testing. Following treatment with a serial dilution of antifungal drug and staining with a fluorescent dye, fungal cells are measured for their fluorescence using a flow cytometer. Alterations in fluorescence are interpreted as changes in cell viability and thus an indication of antifungal-induced damage. The MIC may be defined as the lowest concentration of a specific antifungal drug to which the percentage of positive cells, or cells showing high fluorescence, is above a threshold value, compared to an isolate with a known in vitro susceptibility. Various fluorochromes are available for use as dyes, such as propidium iodide (PI), acridine orange (AO), ethidium bromide, and 2-choro-4-[2,3-dihydro-3-methyl-[benzo-1,3-thiazol-2-yl]-methyl-idene]-1 phenylquinolinium iodide (FUN-1) (104–110).
Comparisons of this method to reference AFST methods have been focused on Candida spp. and have resulted in promising data for multiple species and multiple antifungals (107, 109, 111). However, in spite of high overall EA and categorical agreement with the CLSI method, very major errors have been reported with flow cytometry, especially with C. krusei (112). Also, while results can be generated in a few hours as opposed to at least a day for broth microdilution, FACS as it stands now is labor-intensive, requires a high level of technician expertise, and would be impractical in resource-limited settings.
MALDI-TOF Mass Spectrometry
Matrix-assisted laser desorption ionization–time of flight (MALDI-TOF) mass spectrometry detects protein profiles directly from clinical isolates and has become a staple in the clinical microbiology laboratory for identification of both bacteria and fungi (113). This technology has been investigated for application in AFST as well. In general, an isolate is exposed to a concentration of antifungal drug and analyzed via MALDI-TOF mass spectrometry, and any changes to the proteome compared to a drug-free control are interpreted as an indicator of antifungal-induced damage (114). An early study investigating this application tested Candida albicans with serial dilutions of fluconazole and found that the lowest concentration of drug that induced a significant change in the mass spectrum profile, termed the minimal profile change concentration (MPCC), correlated well with the MIC as determined by CLSI methodology (115). Other results have been mixed. This method has been applied to other species of Candida and Aspergillus against caspofungin and found to have complete EA with a reference method as well as up to 94.1% categorical agreement, although agreement was better for susceptible than resistant isolates (116, 117). However, another study found a wide range of EA values between MALDI-TOF mass spectrometry and CLSI method results (54 to 97%, depending on the species), with a reproducibility of results as low as 54% (118). Modifications have been made to make this method more rapid and simple; this modified method is reportedly able to detect 90.1% of FKS1 mutants of C. albicans but does not detect FKS2 mutations in C. glabrata (119, 120). Another study of MALDI-TOF AFST with echinocandins and Aspergillus species showed good correlation with broth microdilution, but because of the extended incubation time, it concluded that there was no advantage to MALDI-TOF mass spectrometry (121).
Potential advantages of developing MALDI-TOF mass spectrometry for use in susceptibility testing are the elimination of subjectivity present in the visual readout as described by current CLSI methodology, the elimination of long turnaround times, and a reduction in the burden posed by trailing isolates of Candida, the latter of which is also an advantage shared by the Vitek 2 and the EUCAST reference methods for yeasts. Laboratories which already house the instrument for identification of isolates might appreciate the value of a dual-use method for AFST as well. But additional study is necessary before widespread implementation in mainstream clinical laboratories.
Agar-Based Antifungal Screening
Screening fungal isolates for their ability to grow on standard agar containing antifungal agents is a useful strategy in a variety of settings. One such technology, X-Plate, is a combination identification-susceptibility testing assay that utilizes Candida CHROMagar (Becton, Dickinson and Company, Sparks, MD, USA) poured into four quadrants of a petri plate. Each of the four distinct quadrants also contains a different concentration of fluconazole: 0 μg/ml, 8 μg/ml, 16 μg/ml, or 64 μg/ml. This system allows for the presumptive identification of a Candida species directly from a clinical specimen via the color of the colony as well as categorization of fluconazole resistance via the presence of drug. When tested with C. albicans, C. glabrata, C. parapsilosis, and C. tropicalis of various susceptibility profiles, X-Plate showed 100% concordance with CLSI broth microdilution (122). It was also evaluated as a high-throughput screen of Candida spp., with results reported to be comparable to other studies of Candida susceptibility (122). In addition to this product, there are a few reports of similar, “in-house-created” plates that use antifungal drugs as a component of Candida CHROMagar (123–125).
Another commercial example of agar-based antifungal screening is the VIPcheck assay (Mediaproducts BV, The Netherlands). This product consists of four wells of RPMI agar, each containing a different azole antifungal, i.e., 4 μg/ml itraconazole, 2 μg/ml voriconazole, and 0.5 μg/ml posaconazole, and one growth control well. This assay has been used in surveillance studies for the detection of azole resistance in Aspergillus fumigatus and offers a rapid, simple screen for antifungal resistance with sensitivity ranging from 92 to 100% and specificity ranging from 67 to 100% (55, 56, 126). This product was the basis for the establishment of EUCAST E.DEF 10.1 as described above.
PAO-Based Culture
Porous aluminum oxide (PAO) is a honeycomb-like material composed of cylindrical pores arranged in a hexagonal array. As this material can be created to self-assemble into ordered configurations, it has a broad range of applications as a scaffold in nanotechnology (127). PAO has been investigated as a novel method of measuring drug resistance. In general, an isolate is subcultured onto a strip of PAO that is placed on an RPMI plate containing a specific concentration of antifungal drug. After a period of time, the PAO strip is analyzed microscopically, and any change in microcolony area is associated with drug activity or inactivity (128–131).
For amphotericin B, the echinocandins, and triazoles, this assay demonstrated essential agreement (EA) of 88.2%, 91.2%, and 79.4 to 82.4%, respectively, with the EUCAST reference method. Moreover, results were ready after approximately 3.5 to 7 h as opposed to 24 h for traditional testing. This technology could be automated for ease of use in routine testing and adapted to other fungi.
IMC
Isothermal microcalorimetry (IMC) is the measure of heat flow in response to a chemical. In the context of AFST, microcalorimetry is a method by which the precise amount of heat production, as it relates to microbial metabolism, is measured and then related to changes in microbial growth. In short, vials containing the organism, growth medium, and serial dilutions of antimicrobial drug are housed within an IMC instrument, and the lowest concentration of drug which inhibits the total heat produced by the growth control to a specified degree is calculated and termed the MHIC, or minimal heat inhibitory concentration. (132–134).
Two studies performed with Aspergillus spp. reported the EA between the MHIC and the MIC/MEC as defined by CLSI as 90% for amphotericin B, 100% for voriconazole, 90% for posaconazole, and 70% for caspofungin and correctly distinguished between voriconazole-susceptible and -resistant isolates (135, 136). This technology has also been applied to other molds, including Fusarium solani, Lomentospora prolificans, Scedosporium apiospermum, Rhizopus arrhizus, Rhizomucor pusillus, and Lichtheimia corymbifera, with a similar high degree of correlation to MIC data by reference method (137). EA with broth microdilution was shown for Candida species with anidulafungin, caspofungin, and micafungin, but there were major discrepancies for C. glabrata and C. parapsilosis with fluconazole (138). In theory, this method could be automated for high-throughput use in routine clinical testing and for testing of novel antifungal drugs.
Validity of AFST Nonstandardized Testing Systems
The central purpose of susceptibility testing is detection of antifungal resistance. Unfortunately, when the validity of various testing systems is analyzed, most isolates used are drug susceptible, a result of the overall rarity of antifungal resistance among clinical isolates. As a result, there is an abundance of data to show that susceptible isolates are identified as susceptible but comparatively little data showing that resistant isolates are identified as resistant. When studies are performed that use an abundance of resistant isolates, errors become more apparent (100). In an effort to begin to alleviate this problem and allow a more robust validation of nonstandardized systems, the U.S. Centers for Disease Control and Prevention (CDC) and the Food and Drug Administration (FDA) have developed the CDC/FDA Antibiotic Resistance Isolate Bank, where isolate panels that contain resistant isolates can be ordered free of charge (https://www.cdc.gov/drugresistance/resistance-bank/index.html).
INTERPRETATION OF MIC DATA
An MIC is an artificially derived in vitro measure of drug activity. The real value of the MIC lies in its ability to predict the clinical outcome of treatment based on an established breakpoint. Breakpoints themselves are developed by considering several factors. The first factor is the MIC value (or the range of MIC values) encompassed by the wild-type isolates of a given fungal-antifungal combination, known as the MIC distribution. MIC values for isolates with known mechanisms of resistance are very useful for validating the endpoint of the wild-type distribution but are not essential. The second factor is the pharmacokinetics/pharmacodynamics of the antifungal in vivo. This reflects the ability to deliver the antifungal and have it reach the site of infection at the desired concentration. Drug-drug interactions and the host’s ability to process the antifungal and maintain high enough trough levels play an important role. Overall, the health and immune status of the host play a very important role in the outcome of treatment. Clinical trials are used to consider all these factors and to determine the highest MIC value that also allows a favorable outcome for the patient. It should be noted that antifungal resistance as measured in vitro will not necessarily translate to treatment failure in a patient; AFST cannot uniformly predict success or failure. Infections due to susceptible isolates respond to therapy approximately 90% of the time, while infections due to resistant isolates respond to therapy approximately 60% of the time (139). The MIC is only one factor that should be considered as a part of a larger, often complicated, clinical picture.
Even when a standardized protocol is used, results can be influenced by subtle variations in inoculum size, growth phase of the cells, incubation temperature, and incubation time. In most standards, these components of testing are given as a range of possibilities (for instance, temperature is given as 35 ± 2°C). When reading visually, the endpoint can also be influenced by individual interpretation. This translates as a range of possible MIC values for any given isolate, all of them being technically and essentially correct (13, 30, 81, 140, 141). In general, if the MIC value of a single isolate is determined daily for 30 days, the result will be a range of MIC values, usually encompassing a modal or central MIC and 1 log2 dilution on either side. This is the reason that the MIC values for QC isolates are given as a range of values rather than as a single value (5, 27). The modal MIC of QC isolates should fall on the midvalue range; that is the QC target. Practically, this means that two technicians can read the same isolate at 2 log2 dilutions apart and yet their MIC values will be in essential agreement (within 2 dilutions of each other) while not necessarily in categorical agreement (agreement by category of susceptible, intermediate, or resistant). However, the MIC agreement between two technicians over time should be more similar than dissimilar whether they are in essential agreement or not.
Challenges to Interpreting Broth Microdilution
There are in vitro phenomena which complicate the endpoint determination, independent of the standard being followed, and may cause a determination of false resistance. One example is the phenomenon of trailing growth (142–144). The drug class of fungistatic azoles, such as fluconazole, voriconazole, and itraconazole, incompletely inhibit the growth of some species of Candida in vitro likely due to the activation of the stress response pathways. Although trailing isolates appear to grow at high drug concentrations, they remain susceptible to the drug (144). When visualized in a broth microdilution assay, this phenomenon appears as reduced yet persistent microbial growth beyond the MIC cutoff point. Trailing growth may present difficulties when reading the results visually, as defined by the CLSI, and may be misinterpreted as drug resistance. It is most commonly observed with C. albicans and C. tropicalis but for other species as well (145). Another example of a “trailing” growth phenomenon or incomplete growth inhibition is the effect of the echinocandins on mold species. Growth is not inhibited but rather changed, and so interpretation becomes subjective (146).
A second scenario, the paradoxical effect, can obstruct the appropriate reading of broth microdilution as well, especially for caspofungin and some Candida species (147–149). Instead of consistent growth across the wells, as seen in trailing growth, there is a distinct drop-off in growth and then a distinct regrowth further up the concentration gradient (Fig. 2) (150, 151). This phenomenon is also called the Eagle effect, so named after being first described by Harry Eagle (152). The mechanism underlying this phenomenon has been attributed to alteration in cell wall content and structure in the fungi as a compensatory response to antifungal drug stress. A decrease in cell wall proteins β-1,3-glucan and β-1,6-glucan and an increase in cell wall chitin content are the most frequently indicated sources of the regrowth (153). As shown for trailing growth, there is no clear connection between in vitro paradoxical growth of an isolate and in vivo resistance (154).
FIG 2.

Example of paradoxical growth phenomenon. There is complete clearing of the wells after the MIC point for anidulafungin, but there is a distinct drop-off in growth and then a distinct regrowth further up the concentration gradient for caspofungin, as indicated with an asterisk.
Established Breakpoints
Breakpoints are the most reliable tool to link an MIC value generated by in vitro testing to the most likely in vivo response of that isolate to an achievable concentration of drug. Breakpoints differentiate isolates with a high likelihood of treatment success from those which are more likely to fail.
Both the CLSI and EUCAST have defined breakpoints for several antifungal drug-fungal species combinations. As discussed above, both groups consider an assortment of information: distributions of MIC values, molecular markers of drug resistance, pharmacokinetic and pharmacodynamics data, and the relationship between patient outcome and MIC value. EUCAST also considers the most common dosage of a particular antifungal and the definition of wild-type population at the species level when setting breakpoints. Although the methods of generating the MIC values and the approaches to establishing breakpoints are slightly different, the breakpoints defined by the two consensus groups are strikingly similar.
Table 3 details the current breakpoints for each antifungal drug against both yeasts and molds. Breakpoints exist for the most frequently encountered fungi in the clinical laboratory. The CLSI lists breakpoints for the most common Candida species to anidulafungin, caspofungin, micafungin, fluconazole, and voriconazole (155–157). There are no breakpoints for fluconazole against C. krusei, as this species is assumed to be intrinsically resistant. Although there is significant outcome data for Candida guilliermondii and the echinocandins, there is not enough outcome data to demonstrate a correlation between MIC value and clinical outcome for fluconazole and voriconazole against C. guilliermondii. For C. glabrata and voriconazole, there is not a clear correlation between outcome and MIC value, so there is no breakpoint for that combination. Due to the paucity of clinical outcome data, the CLSI has not yet established breakpoints for any molds, with a single exception: the CLSI Subcommittee on Antifungal Susceptibility Tests passed breakpoints for voriconazole against A. fumigatus in early 2019 (8). These will be published in the next edition of the M61 document in early 2020.
TABLE 3.
Current antifungal clinical breakpoints and interpretive categories for yeasts and molds via 2019 CLSI and EUCAST standards of broth microdilutiona
| Antifungal agent | Species | CLSI breakpoint (μg/ml) |
EUCAST breakpoint (mg/liter) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| S | I | SDD | R | S | I | SDD | R | ||
| Anidulafungin | C. albicans | ≤0.25 | 0.5 | — | ≥1 | ≤0.032 | — | — | >0.032 |
| C. glabrata | ≤0.12 | 0.25 | — | ≥0.5 | ≤0.064 | — | — | >0.064 | |
| C. guilliermondii | ≤2 | 4 | — | ≥8 | — | — | — | — | |
| C. krusei | ≤0.25 | 0.5 | — | ≥1 | ≤0.064 | — | — | >0.064 | |
| C. parapsilosis | ≤2 | 4 | — | ≥8 | ≤0.002 | — | — | >4 | |
| C. tropicalis | ≤0.25 | 0.5 | — | ≥1 | ≤0.064 | — | — | >0.064 | |
| Caspofungin | C. albicans | ≤0.25 | 0.5 | — | ≥1 | — | — | — | — |
| C. glabrata | ≤0.12 | 0.25 | — | ≥0.5 | — | — | — | — | |
| C. guilliermondii | ≤2 | 4 | — | ≥8 | — | — | — | — | |
| C. krusei | ≤0.25 | 0.5 | — | ≥1 | — | — | — | — | |
| C. parapsilosis | ≤2 | 4 | — | ≥8 | — | — | — | — | |
| C. tropicalis | ≤0.25 | 0.5 | — | ≥1 | — | — | — | — | |
| Micafungin | C. albicans | ≤0.25 | 0.5 | — | ≥1 | ≤0.016 | — | — | >0.016 |
| C. glabrata | ≤0.06 | 0.12 | — | ≥0.25 | ≤0.032 | — | — | >0.032 | |
| C. guilliermondii | ≤2 | 4 | — | ≥8 | — | — | — | — | |
| C. krusei | ≤0.25 | 0.5 | — | ≥1 | — | — | — | — | |
| C. parapsilosis | ≤2 | 4 | — | ≥8 | ≤0.002 | — | — | >2 | |
| C. tropicalis | ≤0.25 | 0.5 | — | ≥1 | — | — | — | — | |
| Voriconazole | C. albicans | ≤0.12 | 0.25–0.5 | — | ≥1 | ≤0.064 | — | — | >0.25 |
| C. krusei | ≤0.5 | 1 | — | ≥2 | — | — | — | — | |
| C. parapsilosis | ≤0.12 | 0.25–0.5 | — | ≥1 | ≤0.125 | — | — | >0.25 | |
| C. tropicalis | ≤0.12 | 0.25–0.5 | — | ≥1 | ≤0.125 | — | — | >0.25 | |
| C. dubliniensis | — | — | — | — | ≤0.064 | — | — | >0.25 | |
| A. fumigatus | —b | —b | —b | —b | ≤1 | — | — | >2 | |
| Fluconazole | C. albicans | ≤2 | — | 4 | ≥8 | ≤2 | — | — | >4 |
| C. glabrata | — | — | ≤32 | ≥64 | ≤0.002 | — | — | >32 | |
| C. parapsilosis | ≤2 | — | 4 | ≥8 | ≤2 | — | — | >4 | |
| C. tropicalis | ≤2 | — | 4 | ≥8 | ≤2 | — | — | >4 | |
| Non-species specific | — | — | — | — | ≤2 | — | — | >4 | |
| Itraconazole | C. albicans | — | — | — | — | ≤0.064 | — | — | >0.064 |
| C. dubliniensis | — | — | — | — | ≤0.064 | — | — | >0.064 | |
| C. parapsilosis | — | — | — | — | ≤0.125 | — | — | >0.25 | |
| C. tropicalis | — | — | — | — | ≤0.125 | — | — | >0.25 | |
| A. flavus | — | — | — | — | ≤1 | — | — | >2 | |
| A. fumigatus | — | — | — | — | ≤1 | — | — | >2 | |
| A. nidulans | — | — | — | — | ≤1 | — | — | >2 | |
| A. terreus | — | — | — | — | ≤1 | — | — | >2 | |
| Isavuconazole | A. fumigatus | — | — | — | — | ≤1 | — | — | >1 |
| A. nidulans | — | — | — | — | ≤0.25 | — | — | >0.25 | |
| A. terreus | — | — | — | — | ≤1 | — | — | >1 | |
| Posaconazole | C. albicans | — | — | — | — | ≤0.064 | — | — | >0.064 |
| C. dubliniensis | — | — | — | — | ≤0.064 | — | — | >0.064 | |
| C. parapsilosis | — | — | — | — | ≤0.064 | — | — | >0.064 | |
| C. tropicalis | — | — | — | — | ≤0.064 | — | — | >0.064 | |
| A. fumigatus | — | — | — | — | ≤0.125 | — | — | >0.25 | |
| A. terreus | — | — | — | — | ≤0.125 | — | — | >0.25 | |
| Amphotericin B | C. albicans | — | — | — | — | ≤1 | — | — | >1 |
| C. glabrata | — | — | — | — | ≤1 | — | — | >1 | |
| C. krusei | — | — | — | — | ≤1 | — | — | >1 | |
| C. parapsilosis | — | — | — | — | ≤1 | — | — | >1 | |
| C. tropicalis | — | — | — | — | ≤1 | — | — | >1 | |
| A. fumigatus | — | — | — | — | ≤1 | — | — | >2 | |
| A. niger | — | — | — | — | ≤1 | — | — | >2 | |
CLSI and EUCAST breakpoints are reported in different units. Em dashes represent instances where breakpoints have not been established. S, susceptible; I, intermediate; SDD, susceptible dose dependent; R, resistant.
The CLSI Subcommittee on Antifungal Susceptibility Tests passed breakpoints for voriconazole against A. fumigatus in early 2019. These will be published in the next edition of the M61 document in early 2020 (8).
The available breakpoints from EUCAST for Candida spp. (158) differ from those established by the CLSI (Table 3). Version 9 of the EUCAST breakpoint tables for interpretation of MICs includes itraconazole and posaconazole breakpoints for several species, including C. albicans, C. dubliniensis, C. parapsilosis, and C. tropicalis (10, 159, 160). There are also voriconazole breakpoints available for C. dubliniensis. No breakpoints are published by EUCAST for caspofungin against any Candida spp. due to significant interlaboratory variation in modal MIC range. Unlike the CLSI, EUCAST lists breakpoints for amphotericin B against C. albicans, C. glabrata, C. krusei, and C. tropicalis (161). Also, unlike the CLSI, there are no EUCAST breakpoints for voriconazole or micafungin against C. krusei or for micafungin against C. tropicalis due to insufficient evidence of adequate correlation. EUCAST also provides non-species-related breakpoints for fluconazole for use with organisms without specific breakpoints.
In addition to the Candida species, EUCAST has established Aspergillus species breakpoints for amphotericin B and several triazoles. This includes breakpoints for amphotericin B against A. fumigatus and A. niger, isavuconazole breakpoints against A. fumigatus, A. nidulans, and A. terreus, itraconazole breakpoints against A. flavus, A. fumigatus, A. nidulans, and A. terreus, posaconazole breakpoints against A. fumigatus and A. terreus, and voriconazole breakpoints against A. fumigatus (10–12). The remaining species-antifungal drug combinations have insufficient evidence of adequate correlation data. No breakpoints are listed for an echinocandin against any Aspergillus spp.
EUCAST is currently revising their breakpoints to encompass a change to the previous category of “I” or intermediate, which is now revised to “susceptible, increased exposure.” In addition, EUCAST has introduced an additional category of “area of technical uncertainty,” or ATU, which will help the laboratory consider other factors besides the MIC value for assigning a category. These changes now appear on the EUCAST website when they are implemented (http://www.eucast.org/clinical_breakpoints/).
Establishment of Epidemiological Cutoff Values (ECVs or ECOFFs)
One of the limitations to the establishment of breakpoints is the paucity of MIC-clinical trial outcome relationship data. Even for the most common fungal pathogens, controlled treatment and outcome data may be scarce. For rare fungal pathogens, robust data are even more challenging to collect, with too few clinical cases resulting in too few data points. Further complicating the issue, attributable mortality may be difficult to confirm with fungal pathogens in general, as the patients who are most likely to suffer from fungal infections often have other severe comorbidities. Taken together, there will likely never be clinical breakpoints for certain less common fungal pathogens or for certain fungus-antifungal drug combinations. In these instances, defining the epidemiological cutoff value (ECV [CLSI term] or ECOFF [EUCAST term]) is a helpful alternative.
An ECV is the MIC or MEC that separates a population of isolates into those with or without acquired or mutational resistance by defining the upper limit of the wild-type MIC/MEC distribution. It is not a breakpoint and should not be used as such or considered an equivalent. Unlike a breakpoint, an ECV does not necessarily predict clinical success or failure of a particular antifungal but rather predicts whether a specific isolate carries resistance to a drug which is known to otherwise have activity against that species. It has no utility for species which are inherently resistant to an antifungal, such as C. krusei and fluconazole.
To establish an ECV, an MIC distribution is the most important criterion (162). By the standards established in CLSI document M57, 100 isolates from at least three different laboratories are needed (163). Once an ECV is established, it can be used to help guide treatment decisions. An isolate whose MIC falls below the determined ECV is likely wild type and should respond to therapy, as would other wild-type isolates of that species. That drug may be considered by a clinician for treatment if it is normally used for that species. On the other hand, an isolate whose MIC falls above the ECV is likely non-wild type and may have acquired resistance to that drug. In this circumstance, the clinician should exercise caution with that drug and consider other treatment options. The other piece of information conveyed by the ECV is the general susceptibility of an isolate to an antifungal. As the ECV is the end of the wild-type MIC distribution, it conveys the general range of MICs for any given fungus-antifungal combination and when combined with knowledge of the pharmacokinetics/pharmacodynamics may allow a clinician to decide whether an achievable dose below the ECV is possible.
The CLSI specifies the principles behind and the procedures for the development of ECVs for antifungal testing in document M57 and lists available ECVs themselves in document M59 (163, 164). At this time, EUCAST ECOFFs for fungi can be found on the EUCAST website under the appropriate rationale document (http://www.eucast.org/astoffungi/rationale_documents_for_antifungals/) (Table 4).
TABLE 4.
Current epidemiological cutoff values for yeasts and molds via 2019 CLSI and EUCAST standards of broth microdilutiona
| Antifungal agent | Species | CLSI ECV (μg/ml) | EUCAST ECOFF (mg/liter) |
|---|---|---|---|
| Anidulafungin | C. albicans | — | 0.032 |
| C. glabrata | — | 0.064 | |
| C. krusei | — | 0.064 | |
| C. parapsilosis | — | 4 | |
| C. tropicalis | — | 0.064 | |
| C. dubliniensis | 0.12 | — | |
| C. lusitaniae | 1 | — | |
| Micafungin | C. albicans | — | 0.016 |
| C. glabrata | — | 0.032 | |
| C. krusei | — | 0.25 | |
| C. parapsilosis | — | 2 | |
| C. tropicalis | — | 0.064 | |
| C. dubliniensis | 0.12 | — | |
| C. lusitaniae | 0.5 | — | |
| Voriconazole | C. albicans | — | 0.125 |
| C. glabrata | — | 1 | |
| C. guilliermondii | — | 0.25 | |
| C. krusei | — | 1 | |
| C. parapsilosis | — | 0.125 | |
| C. tropicalis | — | 0.125 | |
| C. lusitaniae | — | 0.064 | |
| A. flavus | — | 2 | |
| A. fumigatus | — | 1 | |
| A. niger | — | 2 | |
| A. terreus | — | 2 | |
| Fluconazole | C. albicans | — | 1 |
| C. glabrata | — | 32 | |
| C. guilliermondii | — | 16 | |
| C. krusei | — | 128 | |
| C. parapsilosis | — | 2 | |
| C. tropicalis | — | 2 | |
| Itraconazole | C. albicans | — | 0.064 |
| C. glabrata | 4 | 2 | |
| C. guilliermondii | — | 2 | |
| C. lusitaniae | 1 | 0.125 | |
| C. krusei | 1 | 1 | |
| C. parapsilosis | — | 0.125 | |
| C. tropicalis | 0.5 | 0.125 | |
| C. dubliniensis | — | 0.064 | |
| C. guilliermondii | — | 2 | |
| A. flavus | — | 1 | |
| A. fumigatus | — | 1 | |
| A. nidulans | — | 1 | |
| A. niger | — | 4 | |
| A. terreus | — | 0.5 | |
| Isavuconazole | A. flavus | — | 2 |
| A. fumigatus | — | 2 | |
| A. nidulans | — | 0.25 | |
| A. niger | — | 4 | |
| A. terreus | — | 1 | |
| Amphotericin B | C. albicans | 2 | 1 |
| C. glabrata | 2 | 1 | |
| C. krusei | 2 | 1 | |
| C. parapsilosis | 2 | 1 | |
| C. tropicalis | 2 | 1 |
CLSI and EUCAST breakpoints are reported in different units. Em dashes represent instances where ECVs have not been established.
Besides publishing ECOFFs, EUCAST also collects data for MIC/MEC distributions for fungus-antifungal combinations and makes those data available (http://www.eucast.org/mic_distributions_and_ecoffs/). While these data are not useful for predicting clinical outcomes for patients, they may have some usefulness for clinical decision-making. First, these data are the basis for the establishment of ECOFFs. It takes a large amount of data to make accurate ECOFFs, and except for a few fungal species, the clinical cases are few and far between. With the continual collection of distribution data, we come closer to the establishment of an ECOFF. Second, while not useful for prediction outcome, the distribution range, like the ECV, allows the clinician to at least make a prediction of whether an achievable concentration in the target organ can be established. A distribution of MIC values at the lower end of the achievable serum concentration indicates that an antifungal will be more likely to work than a distribution that is at the higher end of the achievable dose. For the distribution to be useful, some knowledge of the pharmacokinetic/pharmacodynamic (PK/PD) range must be known and a pharmacy consult may be useful.
FUTURE OF ANTIFUNGAL SUSCEPTIBILITY TESTING
Reporting AFST Results to Clinicians
An important consideration in AFST is what to report to the clinician. For many fungus-antifungal combinations with no breakpoints or ECVs, the only thing that can be reported is the MIC value. But where there are breakpoints available, there is some debate over whether to simply report the interpretive category or whether to provide both the interpretation and the MIC value. There is some value in providing an MIC. A good example is Candida glabrata and fluconazole. There is no susceptible category for this combination, only susceptible dose-dependent (SDD) and resistant. The MIC distribution for SDD isolates ranges up to ≤32 μg/ml. While both 2 μg/ml and 32 μg/ml are in the same category, 16 μg/ml and 32 μg/ml are both in essential agreement or standard error range of the resistant breakpoint of 64 μg/ml. A clinician who would like to take a patient off an echinocandin is more likely to use azole step-down therapy on a C. glabrata infection if the initial MIC value for the isolate was 2 μg/ml rather than 16 or 32 μg/ml, and only by providing the MIC value could that decision be made. This would be especially relevant in an institution that is already experiencing a high number of cases of echinocandin-resistant C. glabrata infections (165, 166).
The reporting of ECVs poses a similar problem. Most laboratories have not yet embraced the reporting of fungal ECVs, and that is partly due to inexperience in conveying their meaning within a larger context. By providing the ECV, at least some frame of reference for the MIC value has been provided (164). For those fungi with antifungal ECVs, the clinician can discern whether the fungus is wild type or whether it is probable that it has developed resistance (167).
A typical report might say “The MIC for this isolate of Candida kefyr against micafungin is 0.06 μg/ml. The ECV for Candida kefyr against micafungin is 0.125 μg/ml. This isolate is likely wild type, and micafungin may be considered a treatment option.” To make use of the ECV, as stated above, some knowledge of the PK/PD should be known, and a pharmacy or infectious disease consult should be considered.
Trends in Antifungal Resistance
The emergence of antifungal drug resistance, whether to a single drug class or to multiple antifungals, is an impediment to the management of IFIs. With only a short list of systemically available antifungals from which to choose, clinicians are already limited for choice in antifungal therapy. That choice may be further complicated by drug-drug interactions and/or side effects like toxicity, which are known to occur with antifungal drugs (168). Antifungal resistance may be the result of long-term exposure to the drug but may also be an intrinsic quality of a particular Candida or mold species. So, while the rate of microbiological resistance to antifungal drugs is lower than that for antibacterial drugs, it still represents a major clinical challenge (169). Understanding when it occurs by measuring it in vitro is essential to preserving antifungal drug effectiveness.
Candida species are among the most frequent causes of IFIs, and although clinically available antifungal agents still show high activity against them, species-specific resistance has been reported worldwide. In the United States and parts of Europe, including Norway and Switzerland, the incidence of fluconazole resistance for Candida albicans, Candida tropicalis, and Candida parapsilosis is low, at approximately 2%, 5%, and 4%, respectively. Resistance to the echinocandin drug class is nearly nonexistent in these regions for the same species, at <1% (170–172). Similar rates of fluconazole resistance are observed in Asia-Pacific and India, with slightly higher resistance rates seen for C. tropicalis and C. parapsilosis (173, 174). Fluconazole resistance in C. tropicalis has been observed with higher frequency in Taiwan, Australia, and Belgium, at 11%, 17%, and 20%, respectively (175–177). Candida krusei is considered intrinsically resistant to fluconazole but shows high susceptibility to the echinocandins, with 100% of isolates being susceptible in a recent global SENTRY report (178).
The landscape for Candida glabrata is quite different from that for other Candida species. Recent population-based surveillance in the United States identified fluconazole resistance in 10% of C. glabrata isolates (170). Worldwide rates of fluconazole resistance for C. glabrata, as collected by SENTRY and ARTEMIS antifungal surveillance programs, are reported as 10.2% and 11.9%, respectively (47, 178). Resistance to the echinocandins is also higher for C. glabrata than for other species of Candida in the United States and, depending on institutional differences, can range from 0 to 4% or as high as 10% (165, 179, 180). Echinocandin susceptibility patterns in other parts of the world vary for C. glabrata. For example, Germany and Austria report low echinocandin resistance among C. glabrata isolates (<1%) (181). This is echoed in SENTRY data, which published rates of between 0.4 and 1.9% for Europe, 0 and 1.7% for Asia-Pacific, and 0% for Latin America (178). Echinocandin resistance in C. glabrata is often observed in conjunction with azole resistance, resulting in multidrug-resistant isolates. This occurs in 9% of echinocandin-resistant isolates in the United States (170).
The emerging pathogen Candida auris is unique in many ways compared to other species of Candida, and one defining characteristic is unprecedented resistance to antifungal drugs. Breakpoints have not yet been established for C. auris, but tentative breakpoints have been proposed based on wild-type distribution, molecular mutation detection, and PK/PD modeling in mice (https://www.cdc.gov/fungal/candida-auris/c-auris-antifungal.html) (182). Although the patterns of susceptibility vary by C. auris clade, this species shows 70% resistance to fluconazole and 5% resistance to echinocandins worldwide. Most striking perhaps though is the 23% resistance observed to amphotericin B, an antifungal to which very few Candida isolates show any remarkable resistance. Twenty percent of C. auris isolates exhibit resistance to two antifungal drug classes, and there are even isolates which are pan-resistant, showing MIC values which are high for all available antifungal drug classes (22, 23).
Aspergillus species are also common agents of IFIs worldwide, and recently, azole resistance in A. fumigatus has garnered global concern (183). This resistance may occur after prolonged azole therapy, as seen with Candida species, but there is overwhelming evidence that environmental use of agricultural azoles cultivates resistance in Aspergillus spp. as well. These environmentally derived resistant isolates are then seen in the clinic as agents of aspergillosis. While azole-resistant aspergillosis was once thought to be infrequent, the epidemiology has changed, with resistance rates increasing dramatically (184–186). It is difficult to calculate the prevalence of azole resistance in A. fumigatus since clinical laboratories do not perform routine susceptibility testing on molds, but azole-resistant isolates have been detected in both the clinic and the environment in Europe, the Middle East, Asia, Africa, Australia, and North and South America (187–194).
Molecular Detection of Resistance
One of the conundrums facing the clinical microbiology laboratory is the increasing frequency of the use of culture-independent diagnostics. Assays such as the SeptiFast (Roche) or the T2Candida (T2 Biosystems) for the detection of Candida species or AsperGenius (PathoNostics, Maastricht, Netherlands) and MycoGENIE (Ademtech, Pessac, France) for the detection of Aspergillus allow fungi to be rapidly and directly detected from blood, but they do not provide an isolate for susceptibility testing (195–198). With the increasing frequency of antifungal resistance, this leaves laboratories in a quandary. For a subset of antifungal-species combinations, the molecular mechanism of antifungal resistance is known. A good example is Candida species and the echinocandins, where resistance has been linked almost exclusively to amino acid mutations in two hot spot regions in the FKS genes (199–201). Molecular assays for the detection of FKS mutations have been developed. These include a molecular beacon assay to detect the S645 mutation in C. albicans (202), a Luminex Mag-Pix assay to detect multiple FKS mutations in C. glabrata (203), and a multiplex melt-curve assay again for the detection of multiple FKS mutations in C. glabrata (204). However, all these assays were developed for isolates and have not been validated for detection of mutations directly from clinical specimens.
Determining the molecular mechanisms of resistance of Candida species to the azoles is even more complicated. For azoles such as fluconazole, the resistance mechanism depends on the species and may be due to one or a combination of several factors: amino acid substitutions in the target gene ERG11, amino acid changes in a transcription factor that causes overexpression of either ERG11 or one of multiple drug transporters, or a mutation in one of the other enzymes in the ergosterol pathway (205, 206).
While there are limitations to the use of molecular detection of resistance, there are settings where it may be efficacious. Azole resistance in A. fumigatus is one situation where rapid detection of resistance would be useful. There are a number of quantitative PCR (qPCR) assays that have been developed to detect various mutations in the CYP51A gene of A. fumigatus, the target of the azoles, directly from clinical specimens (207–209). The two commercially available assays for the detection of azole resistance in A. fumigatus, AsperGenius and MycoGENIE, detect Aspergillus DNA and at the same time are able to detect a limited number of mutations in the CYP51A gene that have been shown to be responsible for azole resistance (197, 198, 210). Although these tests represent a breakthrough in resistance detection, because of gene copy number the sensitivity of detection of A. fumigatus is higher than that for detection of resistance, especially since many resistance mutations are not detected by this assay. This means that although it can sometimes detect resistance, it is not always an accurate predictor of susceptibility. The AsperGenious assay is a multiplex PCR assay for the detection of A. fumigatus, A. terreus, and Aspergillus species but has a second multiplex reaction that detects four mutations (TR34, L98H, Y121F, and T289A) that are responsible for the majority of cases of azole resistance in A. fumigatus (18, 197). The MycoGENIE detects A. fumigatus DNA, but in the same multiplex reaction, it detects TR34 and L98H (198). In a setting such as the Netherlands where the TR34 and L98H and the TR46, Y121F, and T289A mutations are now found in over 10% of all clinical isolates of A. fumigatus, these assays would allow rapid treatment decisions (211). The limitation of these assays is that not all of the mutations that cause azole resistance are detected, and up to 30% of A. fumigatus isolates that are resistant to azoles have no known mechanism of resistance (212).
CONCLUSIONS
There are several reasons why antifungal susceptibility testing is not often performed in the clinical laboratory. Therapeutic choices for antifungals are limited, and so therapy is often empirical. The turnaround time, especially for mold testing, may preclude testing, especially in critical cases. The lack of interpretive criteria for many fungus-antifungal combinations may cause some clinicians to question why to test when they cannot interpret the results. The testing remains an esoteric skill that requires experienced technicians and can be subjective in interpretation. However, there are current recommendations in support of susceptibility testing. In 2012, the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) recommended AFST of all Candida isolates from blood and other deep sites (7). In addition, they stated the usefulness of AFST for isolates from patients who were failing therapy, isolates from rare species, and isolates from species that are known to develop resistance. In 2016 in their revised candidiasis guidelines, the Infectious Diseases Society of America (IDSA) also recommended antifungal susceptibility testing, at least for azoles, for all bloodstream and clinically relevant Candida isolates. They also suggested that echinocandin testing be considered for patients with either C. glabrata or C. parapsilosis infection (6).
The 2016 IDSA guidelines for the management of aspergillosis recommend against routine AFST of Aspergillus isolates (213). Instead, they recommend reserving testing for patients who are unresponsive to antifungal agents or who are suspected to have a resistant isolate. The IDSA guidelines also suggest that AFST can be performed for epidemiological purposes but make no suggestion as to how those data should be used. ESCMID provided the first strong recommendation for susceptibility testing of Aspergillus in 2017 (9). They recommended that AFST be performed on isolates causing invasive disease in patients from regions where resistance is found in surveillance programs. The problem with this recommendation is the paucity of surveillance programs that perform mold susceptibility testing. A stronger recommendation would include language that also recommended the initiation of surveillance programs. The availability of a simple agar growth assay for screening for azole-resistant Aspergillus may allow susceptibility testing to become more commonplace.
We are in an unprecedented era for susceptibility testing. There are two antifungal-resistant species of fungi that are spreading across the globe, multidrug-resistant Candida auris and azole-resistant Aspergillus fumigatus (18, 22, 23, 212). There are also an unprecedented number of new antifungal agents already in phase II or phase III clinical trials (214). In order to adequately treat patients with infections for which there are few agents with clinical efficacy, AFST will have to be more widely available than it currently is. It may soon come to pass that clinicians will have a number of different antifungal classes to choose from for both molds and yeasts. AFST will play a much larger role in the decision-making process for choosing antifungal therapy. In the United States right now, the College of American Pathologists send out a single yeast isolate three times per year for AFST proficiency. There are no programs for mold proficiency. In order to meet the growing needs of patients, clinicians, and clinical laboratories, the overall program of AFST must be expanded.
Biographies

Elizabeth L. Berkow is the Director of the Fungal Reference Laboratory and the Laboratory Team Lead for the Mycotic Diseases Branch at the CDC, where her primary interests are antifungal susceptibility testing and mechanisms of antifungal drug resistance. She also serves the Antibiotic Resistance Laboratory Network (ARLN) at the CDC as a mycology subject matter expert and is a member of the Subcommittee on Antifungal Susceptibility Tests for CLSI. Dr. Berkow has a degree in Clinical Laboratory Science (M.S.) and earned her Ph.D. in Molecular Biology from the University of Tennessee Health Science Center in Memphis, TN. She is an ASCP board-certified medical laboratory scientist.

Shawn R. Lockhart received his Ph.D. from the University of Kentucky and then became a research scientist at the University of Iowa. He became a diplomat of the American Board of Medical Microbiology following a fellowship at the University of Iowa Hospitals and Clinics. Dr. Lockhart joined the CDC in 2008 as the director of the Fungal Reference Laboratory. He is currently Senior Clinical Laboratory Advisor and Senior Advisor for AMR for the Mycotic Diseases Branch at the CDC. He started studying Candida after it was determined to be the cause of death of a close family member. He has been interested in antifungal susceptibility testing since 2006 and has been involved with the Clinical and Laboratory Standards Institute Antifungal Subcommittee since 2009.

Luis Ostrosky-Zeichner, M.D., is a professor of medicine and epidemiology, the Vice-Chair of Medicine for Quality, and the director of the Laboratory of Mycology Research at McGovern Medical School. He obtained his medical degree from Universidad Nacional Autonoma de Mexico. He completed his internal medicine residency at Instituto Nacional de Ciencias Medicas y Nutricion Salvador Zubiran and his infectious diseases fellowship at the University of Texas Medical School at Houston and MD Anderson Cancer Center. He is a fellow of the American College of Physicians, the Infectious Diseases Society of America, the Society of Healthcare Epidemiology of America, and the Academy of the European Confederation of Medical Mycology. He is a board member of the Mycoses Study Group and the International Immunocompromised Host Society. He has advanced training and experience in medical mycology, healthcare epidemiology, transplant infectious diseases, and healthcare quality.
REFERENCES
- 1.Rex JH, Pfaller MA, Galgiani JN, Bartlett MS, Espinel-Ingroff A, Ghannoum MA, Lancaster M, Odds FC, Rinaldi MG, Walsh TJ, Barry AL. 1997. Development of interpretive breakpoints for antifungal susceptibility testing: conceptual framework and analysis of in vitro-in vivo correlation data for fluconazole, itraconazole, and candida infections. Subcommittee on Antifungal Susceptibility Testing of the National Committee for Clinical Laboratory Standards. Clin Infect Dis 24:235–247. doi: 10.1093/clinids/24.2.235. [DOI] [PubMed] [Google Scholar]
- 2.Calhoun DL, Roberts GD, Galgiani JN, Bennett JE, Feingold DS, Jorgensen J, Kobayashi GS, Shadomy S. 1986. Results of a survey of antifungal susceptibility tests in the United States and interlaboratory comparison of broth dilution testing of flucytosine and amphotericin B. J Clin Microbiol 23:298–301. doi: 10.1128/JCM.23.2.298-301.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Galgiani JN, Reiser J, Brass C, Espinel-Ingroff A, Gordon MA, Kerkering TM. 1987. Comparison of relative susceptibilities of Candida species to three antifungal agents as determined by unstandardized methods. Antimicrob Agents Chemother 31:1343–1347. doi: 10.1128/aac.31.9.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Committee for Clinical Laboratory Standards. 1997. Reference method for broth dilution antifungal susceptibility testing of yeasts. National Committee for Clinical Laboratory Standards, Wayne, PA. [Google Scholar]
- 5.Anonymous. 2008. EUCAST definitive document EDef 7.1: method for the determination of broth dilution MICs of antifungal agents for fermentative yeasts. Clin Microbiol Infect 14:398–405. doi: 10.1111/j.1469-0691.2007.01935.x. [DOI] [PubMed] [Google Scholar]
- 6.Pappas PG, Kauffman CA, Andes DR, Clancy CJ, Marr KA, Ostrosky-Zeichner L, Reboli AC, Schuster MG, Vazquez JA, Walsh TJ, Zaoutis TE, Sobel JD. 2016. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis 62:e1–e50. doi: 10.1093/cid/civ933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cuenca-Estrella M, Verweij PE, Arendrup MC, Arikan-Akdagli S, Bille J, Donnelly JP, Jensen HE, Lass-Florl C, Richardson MD, Akova M, Bassetti M, Calandra T, Castagnola E, Cornely OA, Garbino J, Groll AH, Herbrecht R, Hope WW, Kullberg BJ, Lortholary O, Meersseman W, Petrikkos G, Roilides E, Viscoli C, Ullmann AJ. 2012. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: diagnostic procedures. Clin Microbiol Infect 18(Suppl 7):9–18. doi: 10.1111/1469-0691.12038. [DOI] [PubMed] [Google Scholar]
- 8.Subcommittee on Antifungal Susceptibility Tests. January 2019. Antifungal susceptibility testing meeting minutes, January 2019. https://clsi.org/meetings/sub-antifungal/antifungal-susceptibility-testing-files-resources/.
- 9.Ullmann AJ, Aguado JM, Arikan-Akdagli S, Denning DW, Groll AH, Lagrou K, Lass-Florl C, Lewis RE, Munoz P, Verweij PE, Warris A, Ader F, Akova M, Arendrup MC, Barnes RA, Beigelman-Aubry C, Blot S, Bouza E, Bruggemann RJM, Buchheidt D, Cadranel J, Castagnola E, Chakrabarti A, Cuenca-Estrella M, Dimopoulos G, Fortun J, Gangneux JP, Garbino J, Heinz WJ, Herbrecht R, Heussel CP, Kibbler CC, Klimko N, Kullberg BJ, Lange C, Lehrnbecher T, Loffler J, Lortholary O, Maertens J, Marchetti O, Meis JF, Pagano L, Ribaud P, Richardson M, Roilides E, Ruhnke M, Sanguinetti M, Sheppard DC, Sinko J, Skiada A, et al. . 2018. Diagnosis and management of Aspergillus diseases: executive summary of the 2017 ESCMID-ECMM-ERS guideline. Clin Microbiol Infect 24(Suppl 1):e1–e38. doi: 10.1016/j.cmi.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 10.Arendrup MC, Subcommittee on Antifungal Susceptibility Testing (AFST) of the ESCMID European Committee for Antimicrobial Susceptibility Testing (EUCAST), Meletiadis J, Mouton JW, Guinea J, Cuenca-Estrella M, Lagrou K, Howard SJ. 2016. EUCAST technical note on isavuconazole breakpoints for Aspergillus, itraconazole breakpoints for Candida and updates for the antifungal susceptibility testing method documents. Clin Microbiol Infect 22:571.e1–e4. doi: 10.1016/j.cmi.2016.01.017. [DOI] [PubMed] [Google Scholar]
- 11.Hope WW, Cuenca-Estrella M, Lass-Flörl C, Arendrup MC, European Committee on Antimicrobial Susceptibility Testing-Subcommittee on Antifungal Susceptibility Testing . 2013. EUCAST technical note on voriconazole and Aspergillus spp. Clin Microbiol Infect 19:E278–E80. doi: 10.1111/1469-0691.12148. [DOI] [PubMed] [Google Scholar]
- 12.Arendrup MC, Cuenca-Estrella M, Lass-Flörl C, Hope WW, European Committee on Antimicrobial Susceptibility Testing Subcommittee on Antifungal Susceptibility Testing . 2012. EUCAST technical note on Aspergillus and amphotericin B, itraconazole, and posaconazole. Clin Microbiol Infect 18:E248–E250. doi: 10.1111/j.1469-0691.2012.03890.x. [DOI] [PubMed] [Google Scholar]
- 13.Pfaller MA, Bale M, Buschelman B, Lancaster M, Espinel-Ingroff A, Rex JH, Rinaldi MG. 1994. Selection of candidate quality control isolates and tentative quality control ranges for in vitro susceptibility testing of yeast isolates by National Committee for Clinical Laboratory Standards proposed standard methods. J Clin Microbiol 32:1650–1653. doi: 10.1128/JCM.32.7.1650-1653.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pfaller MA, Diekema DJ. 2007. Epidemiology of invasive candidiasis: a persistent public health problem. Clin Microbiol Rev 20:133–163. doi: 10.1128/CMR.00029-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lamoth F, Lockhart SR, Berkow EL, Calandra T. 2018. Changes in the epidemiological landscape of invasive candidiasis. J Antimicrob Chemother 73:i4–i13. doi: 10.1093/jac/dkx444. [DOI] [PubMed] [Google Scholar]
- 16.Wiederhold NP. 2016. Echinocandin resistance in Candida species: a review of recent developments. Curr Infect Dis Rep 18:42. doi: 10.1007/s11908-016-0549-2. [DOI] [PubMed] [Google Scholar]
- 17.Goncalves SS, Souza ACR, Chowdhary A, Meis JF, Colombo AL. 2016. Epidemiology and molecular mechanisms of antifungal resistance in Candida and Aspergillus. Mycoses 59:198–219. doi: 10.1111/myc.12469. [DOI] [PubMed] [Google Scholar]
- 18.Resendiz Sharpe A, ISHAM/ECMM Aspergillus Resistance Surveillance Working Group, Lagrou K, Meis JF, Chowdhary A, Lockhart SR, Verweij PE. 2018. Triazole resistance surveillance in Aspergillus fumigatus. Med Mycol 56:83–92. doi: 10.1093/mmy/myx144. [DOI] [PubMed] [Google Scholar]
- 19.Lestrade PP, Bentvelsen RG, Schauwvlieghe A, Schalekamp S, van der Velden W, Kuiper EJ, van Paassen J, van der Hoven B, van der Lee HA, Melchers WJG, de Haan AF, van der Hoeven HL, Rijnders BJA, van der Beek MT, Verweij PE. 2019. Voriconazole resistance and mortality in invasive aspergillosis: a multicenter retrospective cohort study. Clin Infect Dis 68:1463–1471. doi: 10.1093/cid/ciy859. [DOI] [PubMed] [Google Scholar]
- 20.Schauwvlieghe A, de Jonge N, van Dijk K, Verweij PE, Bruggemann RJ, Biemond BJ, Bart A, von Dem Borne PA, Verbon A, van der Beek MT, Demandt AMP, Oudhuis GJ, Cornelissen JJ, van der Velden W, Span LFR, Kampinga GA, Bruns AH, Vonk AG, Haas PA, Doorduijn JK, Rijnders B. 2018. The diagnosis and treatment of invasive aspergillosis in Dutch haematology units facing a rapidly increasing prevalence of azole-resistance. A nationwide survey and rationale for the DB-MSG 002 study protocol. Mycoses 61:656–664. doi: 10.1111/myc.12788. [DOI] [PubMed] [Google Scholar]
- 21.Kathuria S, Singh PK, Sharma C, Prakash A, Masih A, Kumar A, Meis JF, Chowdhary A. 2015. Multidrug-resistant Candida auris misidentified as Candida haemulonii: characterization by matrix-assisted laser desorption ionization-time of flight mass spectrometry and DNA sequencing and its antifungal susceptibility profile variability by Vitek 2, CLSI broth microdilution, and Etest method. J Clin Microbiol 53:1823–1830. doi: 10.1128/JCM.00367-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chowdhary A, Sharma C, Meis JF. 2017. Candida auris: a rapidly emerging cause of hospital-acquired multidrug-resistant fungal infections globally. PLoS Pathog 13:e1006290. doi: 10.1371/journal.ppat.1006290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lockhart SR, Etienne KA, Vallabhaneni S, Farooqi J, Chowdhary A, Govender NP, Colombo AL, Calvo B, Cuomo CA, Desjardins CA, Berkow EL, Castanheira M, Magobo RE, Jabeen K, Asghar RJ, Meis JF, Jackson B, Chiller T, Litvintseva AP. 2017. Simultaneous emergence of multidrug-resistant Candida auris on 3 continents confirmed by whole-genome sequencing and epidemiological analyses. Clin Infect Dis 64:134–140. doi: 10.1093/cid/ciw691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Galgiant JN, Stevens DA. 1978. Turbidimetric studies of growth inhibition of yeasts with three drugs: inquiry into inoculum-dependent susceptibility testing, time of onset of drug effect, and implications for current and newer methods. Antimicrob Agents Chemother 13:249–254. doi: 10.1128/aac.13.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Galgiani JN, Stevens DA. 1976. Antimicrobial susceptibility testing of yeasts: a turbidimetric technique independent of inoculum size. Antimicrob Agents Chemother 10:721–728. doi: 10.1128/aac.10.4.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arendrup MC, Meletiadis J, Mouton JW, Lagrou K, Hamal P, Guinea J, Subcommittee on Antifungal Susceptibility Testing of the ESCMID European Committee for Antimicrobial Susceptibility Testing . 2017. EUCAST definitive document E.DEF 7.3.1. Method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for yeasts. http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/AFST/Files/EUCAST_E_Def_7_3_1_Yeast_testing__definitive.pdf.
- 27.Clinical and Laboratory Standards Institute. 2017. Reference method for broth dilution antifungal susceptibility testing of yeasts, 4th ed Approved standard M27 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 28.National Committee for Clinical Laboratory Standards. 1992. Reference method for broth dilution antifungal susceptibility testing of yeasts. Proposed standard M27-P. National Committee for Clinical Laboratory Standards, Villanova, PA. [Google Scholar]
- 29.Clinical and Laboratory Standards Institute. 2017. Performance standards for antifungal susceptibility testing of yeasts, 1st ed CLSI supplement M60 Clinical and Laboratory Standards Institute, Wayne PA. [Google Scholar]
- 30.Arendrup MC, Kahlmeter G, Rodriguez-Tudela JL, Donnelly JP. 2009. Breakpoints for susceptibility testing should not divide wild-type distributions of important target species. Antimicrob Agents Chemother 53:1628–1629. doi: 10.1128/AAC.01624-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shields RK, Nguyen MH, Press EG, Updike CL, Clancy CJ. 2013. Caspofungin MICs correlate with treatment outcomes among patients with Candida glabrata invasive candidiasis and prior echinocandin exposure. Antimicrob Agents Chemother 57:3528–3535. doi: 10.1128/AAC.00136-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Espinel-Ingroff A, Arendrup MC, Pfaller MA, Bonfietti LX, Bustamante B, Canton E, Chryssanthou E, Cuenca-Estrella M, Dannaoui E, Fothergill A, Fuller J, Gaustad P, Gonzalez GM, Guarro J, Lass-Florl C, Lockhart SR, Meis JF, Moore CB, Ostrosky-Zeichner L, Pelaez T, Pukinskas SR, St-Germain G, Szeszs MW, Turnidge J. 2013. Interlaboratory variability of caspofungin MICs for Candida spp. using CLSI and EUCAST methods: should the clinical laboratory be testing this agent? Antimicrob Agents Chemother 57:5836–5842. doi: 10.1128/AAC.01519-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pfaller MA, Messer SA, Diekema DJ, Jones RN, Castanheira M. 2014. Use of micafungin as a surrogate marker to predict susceptibility and resistance to caspofungin among 3,764 clinical isolates of Candida by use of CLSI methods and interpretive criteria. J Clin Microbiol 52:108–114. doi: 10.1128/JCM.02481-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pfaller MA, Diekema DJ, Jones RN, Castanheira M. 2014. Use of anidulafungin as a surrogate marker to predict susceptibility and resistance to caspofungin among 4,290 clinical isolates of Candida by using CLSI methods and interpretive criteria. J Clin Microbiol 52:3223–3229. doi: 10.1128/JCM.00782-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clinical and Laboratory Standards Institute. 2017. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi, 3rd ed Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 36.Arendrup MC, Meletiadis J, Mouton JW, Lagrou K, Hamal P, Guinea J, Subcommittee on Antifungal Susceptibility Testing of the ESCMID European Committee for Antimicrobial Susceptibility Testing . 2017. Method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for conidia forming moulds. http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/AFST/Files/EUCAST_E_Def_9_3_1_Mould_testing__definitive.pdf. [DOI] [PubMed]
- 37.Saubolle MA, Hoeprich PD. 1978. Disk agar diffusion susceptibility testing of yeasts. Antimicrob Agents Chemother 14:517–530. doi: 10.1128/aac.14.4.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barry AL, Pfaller MA, Rennie RP, Fuchs PC, Brown SD. 2002. Precision and accuracy of fluconazole susceptibility testing by broth microdilution, Etest, and disk diffusion methods. Antimicrob Agents Chemother 46:1781–1784. doi: 10.1128/aac.46.6.1781-1784.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kirkpatrick WR, Turner TM, Fothergill AW, McCarthy DI, Redding SW, Rinaldi MG, Patterson TF. 1998. Fluconazole disk diffusion susceptibility testing of Candida species. J Clin Microbiol 36:3429–3432. doi: 10.1128/JCM.36.11.3429-3432.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pfaller MA, Barry A, Bille J, Brown S, Ellis D, Meis JF, Rennie R, Rinaldi M, Rogers T, Traczewski M. 2004. Quality control limits for voriconazole disk susceptibility tests on Mueller-Hinton agar with glucose and methylene blue. J Clin Microbiol 42:1716–1718. doi: 10.1128/jcm.42.4.1716-1718.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Clinical and Laboratory Standards Institute. 2009. Method for antifungal disk diffusion susceptibility testing of yeasts; approved guideline, 2nd ed CLSI document M44-A2 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 42.Brown SD, Traczewski MM. 2008. Caspofungin disk diffusion breakpoints and quality control. J Clin Microbiol 46:1927–1929. doi: 10.1128/JCM.00279-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Espinel-Ingroff A, Arthington-Skaggs B, Iqbal N, Ellis D, Pfaller MA, Messer S, Rinaldi M, Fothergill A, Gibbs DL, Wang A. 2007. Multicenter evaluation of a new disk agar diffusion method for susceptibility testing of filamentous fungi with voriconazole, posaconazole, itraconazole, amphotericin B, and caspofungin. J Clin Microbiol 45:1811–1820. doi: 10.1128/JCM.00134-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mendez CC, Serrano MC, Valverde A, Peman J, Almeida C, Martin-Mazuelos E. 2008. Comparison of E-test, disk diffusion and a modified CLSI broth microdilution (M 38-A) method for in vitro testing of itraconazole, fluconazole and voriconazole against dermatophytes. Med Mycol 46:119–123. doi: 10.1080/13693780701670491. [DOI] [PubMed] [Google Scholar]
- 45.Ozkutuk A, Ergon C, Metin DY, Yucesoy M, Polat SH. 2008. Comparison of disk diffusion, E-test and broth microdilution test in determination of susceptibility of Aspergillus species to amphotericin B, itraconazole and voriconazole. J Chemother 20:87–92. doi: 10.1179/joc.2008.20.1.87. [DOI] [PubMed] [Google Scholar]
- 46.Clinical and Laboratory Standards Institute. 2010. Method for antifungal disk diffusion susceptibility testing of nondermatophyte filamentous fungi, approved guideline. CLSI document M51-A Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 47.Pfaller MA, Global Antifungal Surveillance Group, Diekema DJ, Gibbs DL, Newell VA, Ellis D, Tullio V, Rodloff A, Fu W, Ling TA. 2010. Results from the ARTEMIS DISK Global Antifungal Surveillance Study, 1997 to 2007: a 10.5-year analysis of susceptibilities of Candida species to fluconazole and voriconazole as determined by CLSI standardized disk diffusion. J Clin Microbiol 48:1366–1377. doi: 10.1128/JCM.02117-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Meis J, Petrou M, Bille J, Ellis D, Gibbs D. 2000. A global evaluation of the susceptibility of Candida species to fluconazole by disk diffusion. Global Antifungal Surveillance Group. Diagn Microbiol Infect Dis 36:215–223. doi: 10.1016/s0732-8893(99)00152-2. [DOI] [PubMed] [Google Scholar]
- 49.Pfaller MA, Diekema DJ, Messer SA, Boyken L, Hollis RJ. 2003. Activities of fluconazole and voriconazole against 1,586 recent clinical isolates of Candida species determined by broth microdilution, disk diffusion, and Etest methods: report from the ARTEMIS Global Antifungal Susceptibility Program, 2001. J Clin Microbiol 41:1440–1446. doi: 10.1128/jcm.41.4.1440-1446.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pfaller MA, Boyken L, Messer SA, Tendolkar S, Hollis RJ, Diekema DJ. 2005. Comparison of results of voriconazole disk diffusion testing for Candida species with results from a central reference laboratory in the ARTEMIS Global Antifungal Surveillance Program. J Clin Microbiol 43:5208–5213. doi: 10.1128/JCM.43.10.5208-5213.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Arendrup MC, Park S, Brown S, Pfaller M, Perlin DS. 2011. Evaluation of CLSI M44-A2 disk diffusion and associated breakpoint testing of caspofungin and micafungin using a well-characterized panel of wild-type and fks hot spot mutant Candida isolates. Antimicrob Agents Chemother 55:1891–1895. doi: 10.1128/AAC.01373-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pfaller MA, Messer SA, Boyken L, Rice C, Tendolkar S, Hollis RJ, Diekema DJ. 2004. Evaluation of the NCCLS M44-P disk diffusion method for determining susceptibilities of 276 clinical isolates of Cryptococcus neoformans to fluconazole. J Clin Microbiol 42:380–383. doi: 10.1128/jcm.42.1.380-383.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pfaller MA, Hazen KC, Messer SA, Boyken L, Tendolkar S, Hollis RJ, Diekema DJ. 2004. Comparison of results of fluconazole disk diffusion testing for Candida species with results from a central reference laboratory in the ARTEMIS global antifungal surveillance program. J Clin Microbiol 42:3607–3612. doi: 10.1128/JCM.42.8.3607-3612.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pfaller MA, ARTEMIS DISK Global Antifungal Surveillance Group, Boyken L, Hollis RJ, Kroeger J, Messer SA, Tendolkar S, Diekema DJ. 2009. Comparison of results of fluconazole and voriconazole disk diffusion testing for Candida spp. with results from a central reference laboratory in the ARTEMIS DISK Global Antifungal Surveillance Program. Diagn Microbiol Infect Dis 65:27–34. doi: 10.1016/j.diagmicrobio.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 55.Arendrup MC, Verweij PE, Mouton JW, Lagrou K, Meletiadis J. 2018. Multicentre validation of 4-well azole agar plates as a screening method for detection of clinically relevant azole-resistant Aspergillus fumigatus. J Antimicrob Chemother 73:2274. doi: 10.1093/jac/dky218. [DOI] [PubMed] [Google Scholar]
- 56.Buil JB, van der Lee HAL, Rijs A, Zoll J, Hovestadt J, Melchers WJG, Verweij PE. 2017. Single-center evaluation of an agar-based screening for azole resistance in Aspergillus fumigatus by using VIPcheck. Antimicrob Agents Chemother 61:e01250-17. doi: 10.1128/AAC.01250-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Guinea J, Verweij PE, Meletiadis J, Mouton JW, Barchiesi F, Arendrup MC, Subcommittee on Antifungal Susceptibility Testing of the ESCMID European Committee for Antimicrobial Susceptibility Testing (EUCAST) . 2019. How to: EUCAST recommendations on the screening procedure E.Def 10.1 for the detection of azole resistance in Aspergillus fumigatus isolates using four-well azole-containing agar plates. Clin Microbiol Infect 25:681–687. doi: 10.1016/j.cmi.2018.09.008. [DOI] [PubMed] [Google Scholar]
- 58.Pfaller MA, Messer SA, Houston A, Mills K, Bolmstrom A, Jones RN. 2000. Evaluation of the Etest method for determining voriconazole susceptibilities of 312 clinical isolates of Candida species by using three different agar media. J Clin Microbiol 38:3715–3717. doi: 10.1128/JCM.38.10.3715-3717.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pfaller MA, Messer SA, Mills K, Bolmstrom A. 2000. In vitro susceptibility testing of filamentous fungi: comparison of Etest and reference microdilution methods for determining itraconazole MICs. J Clin Microbiol 38:3359–3361. doi: 10.1128/JCM.38.9.3359-3361.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Alexander BD, Byrne TC, Smith KL, Hanson KE, Anstrom KJ, Perfect JR, Reller LB. 2007. Comparative evaluation of Etest and Sensititre YeastOne panels against the Clinical and Laboratory Standards Institute M27-A2 reference broth microdilution method for testing Candida susceptibility to seven antifungal agents. J Clin Microbiol 45:698–706. doi: 10.1128/JCM.01840-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Diekema DJ, Messer SA, Hollis RJ, Boyken LB, Tendolkar S, Kroeger J, Pfaller MA. 2007. Evaluation of Etest and disk diffusion methods compared with broth microdilution antifungal susceptibility testing of clinical isolates of Candida spp. against posaconazole. J Clin Microbiol 45:1974–1977. doi: 10.1128/JCM.02087-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Arendrup M, Lundgren B, Jensen IM, Hansen BS, Frimodt-Møller N. 2001. Comparison of Etest and a tablet diffusion test with the NCCLS broth microdilution method for fluconazole and amphotericin B susceptibility testing of Candida isolates. J Antimicrob Chemother 47:521–526. doi: 10.1093/jac/47.5.521. [DOI] [PubMed] [Google Scholar]
- 63.Pfaller MA, Messer SA, Mills K, Bolmstrom A, Jones RN. 2001. Evaluation of Etest method for determining posaconazole MICs for 314 clinical isolates of Candida species. J Clin Microbiol 39:3952–3954. doi: 10.1128/JCM.39.11.3952-3954.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pfaller MA, Messer SA, Mills K, Bolmstrom A, Jones RN. 2001. Evaluation of Etest method for determining caspofungin (MK-0991) susceptibilities of 726 clinical isolates of Candida species. J Clin Microbiol 39:4387–4389. doi: 10.1128/JCM.39.12.4387-4389.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cuenca-Estrella M, Gomez-Lopez A, Mellado E, Rodriguez-Tudela JL. 2005. Correlation between the procedure for antifungal susceptibility testing for Candida spp. of the European Committee on Antibiotic Susceptibility Testing (EUCAST) and four commercial techniques. Clin Microbiol Infect 11:486–492. doi: 10.1111/j.1469-0691.2005.01166.x. [DOI] [PubMed] [Google Scholar]
- 66.Pfaller MA, Castanheira M, Diekema DJ, Messer SA, Moet GJ, Jones RN. 2010. Comparison of European Committee on Antimicrobial Susceptibility Testing (EUCAST) and Etest methods with the CLSI broth microdilution method for echinocandin susceptibility testing of Candida species. J Clin Microbiol 48:1592–1599. doi: 10.1128/JCM.02445-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Idelevich EA, Groß U, Becker K, Bader O. 2018. Comparative evaluation of different gradient diffusion tests for detection of azole resistance in Aspergillus fumigatus. Diagn Microbiol Infect Dis 91:52–54. doi: 10.1016/j.diagmicrobio.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 68.Al-Hatmi AM, Normand AC, Ranque S, Piarroux R, de Hoog GS, Meletiadis J, Meis JF. 2017. Comparative evaluation of Etest, EUCAST, and CLSI methods for amphotericin B, voriconazole, and posaconazole against clinically relevant Fusarium species. Antimicrob Agents Chemother 61:e01671-16. doi: 10.1128/AAC.01671-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pfaller JB, Messer SA, Hollis RJ, Diekema DJ, Pfaller MA. 2003. In vitro susceptibility testing of Aspergillus spp.: comparison of Etest and reference microdilution methods for determining voriconazole and itraconazole MICs. J Clin Microbiol 41:1126–1129. doi: 10.1128/jcm.41.3.1126-1129.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Espinel-Ingroff A, Rezusta A. 2002. E-test method for testing susceptibilities of Aspergillus spp. to the new triazoles voriconazole and posaconazole and to established antifungal agents: comparison with NCCLS broth microdilution method. J Clin Microbiol 40:2101–2107. doi: 10.1128/jcm.40.6.2101-2107.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Serrano MC, Morilla D, Valverde A, Chávez M, Espinel-Ingroff A, Claro R, Ramírez M, Mazuelos EM. 2003. Comparison of Etest with modified broth microdilution method for testing susceptibility of Aspergillus spp. to voriconazole. J Clin Microbiol 41:5270–5272. doi: 10.1128/jcm.41.11.5270-5272.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Torres-Narbona M, Guinea J, Martínez-Alarcón J, Peláez T, Bouza E. 2007. In vitro activities of amphotericin B, caspofungin, itraconazole, posaconazole, and voriconazole against 45 clinical isolates of zygomycetes: comparison of CLSI M38-A, Sensititre YeastOne, and the Etest. Antimicrob Agents Chemother 51:1126–1129. doi: 10.1128/AAC.01539-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Caramalho R, Maurer E, Binder U, Araújo R, Dolatabadi S, Lass-Flörl C, Lackner M. 2015. Etest cannot be recommended for in vitro susceptibility testing of Mucorales. Antimicrob Agents Chemother 59:3663–3665. doi: 10.1128/AAC.00004-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lamoth F, Alexander BD. 2015. Comparing Etest and broth microdilution for antifungal susceptibility testing of the most-relevant pathogenic molds. J Clin Microbiol 53:3176–3181. doi: 10.1128/JCM.00925-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Law D, Moore CB, Denning DW. 1997. Amphotericin B resistance testing of Candida spp.: a comparison of methods. J Antimicrob Chemother 40:109–112. doi: 10.1093/jac/40.1.109. [DOI] [PubMed] [Google Scholar]
- 76.Pfaller MA, Messer SA, Bolmstrom A. 1998. Evaluation of Etest for determining in vitro susceptibility of yeast isolates to amphotericin B. Diagn Microbiol Infect Dis 32:223–227. doi: 10.1016/s0732-8893(98)00120-5. [DOI] [PubMed] [Google Scholar]
- 77.Peyron F, Favel A, Michel-Nguyen A, Gilly M, Regli P, Bolmström A. 2001. Improved detection of amphotericin B-resistant isolates of Candida lusitaniae by Etest. J Clin Microbiol 39:339–342. doi: 10.1128/JCM.39.1.339-342.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wanger A, Mills K, Nelson PW, Rex JH. 1995. Comparison of Etest and National Committee for Clinical Laboratory Standards broth macrodilution method for antifungal susceptibility testing: enhanced ability to detect amphotericin B-resistant Candida isolates. Antimicrob Agents Chemother 39:2520–2522. doi: 10.1128/aac.39.11.2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lozano-Chiu M, Paetznick VL, Ghannoum MA, Rex JH. 1998. Detection of resistance to amphotericin B among Cryptococcus neoformans clinical isolates: performances of three different media assessed by using E-test and National Committee for Clinical Laboratory Standards M27-A methodologies. J Clin Microbiol 36:2817–2822. doi: 10.1128/JCM.36.10.2817-2822.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Espinel-Ingroff A, Pfaller M, Messer SA, Knapp CC, Killian S, Norris HA, Ghannoum MA. 1999. Multicenter comparison of the Sensititre YeastOne colorimetric antifungal panel with the National Committee for Clinical Laboratory standards M27-A reference method for testing clinical isolates of common and emerging Candida spp., Cryptococcus spp., and other yeasts and yeast-like organisms. J Clin Microbiol 37:591–595. doi: 10.1128/JCM.37.3.591-595.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pfaller MA, Messer SA, Hollis RJ, Espinel-Ingroff A, Ghannoum MA, Plavan H, Killian SB, Knapp CC. 1998. Multisite reproducibility of MIC results by the Sensititre YeastOne colorimetric antifungal susceptibility panel. Diagn Microbiol Infect Dis 31:543–547. doi: 10.1016/s0732-8893(98)00026-1. [DOI] [PubMed] [Google Scholar]
- 82.Morace G, Amato G, Bistoni F, Fadda G, Marone P, Montagna MT, Oliveri S, Polonelli L, Rigoli R, Mancuso I, La Face S, Masucci L, Romano L, Napoli C, Tatò D, Buscema MG, Belli CMC, Piccirillo MM, Conti S, Covan S, Fanti F, Cavanna C, D'Alò F, Pitzurra L. 2002. Multicenter comparative evaluation of six commercial systems and the National Committee for Clinical Laboratory Standards M27-A broth microdilution method for fluconazole susceptibility testing of Candida species. J Clin Microbiol 40:2953–2958. doi: 10.1128/jcm.40.8.2953-2958.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Espinel-Ingroff A, Pfaller M, Messer SA, Knapp CC, Holliday N, Killian SB. 2004. Multicenter comparison of the Sensititre YeastOne colorimetric antifungal panel with the NCCLS M27-A2 reference method for testing new antifungal agents against clinical isolates of Candida spp. J Clin Microbiol 42:718–721. doi: 10.1128/jcm.42.2.718-721.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pfaller MA, Chaturvedi V, Diekema DJ, Ghannoum MA, Holliday NM, Killian SB, Knapp CC, Messer SA, Miskov A, Ramani R. 2008. Clinical evaluation of the Sensititre YeastOne colorimetric antifungal panel for antifungal susceptibility testing of the echinocandins anidulafungin, caspofungin, and micafungin. J Clin Microbiol 46:2155–2159. doi: 10.1128/JCM.00493-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pfaller MA, Chaturvedi V, Diekema DJ, Ghannoum MA, Holliday NM, Killian SB, Knapp CC, Messer SA, Miskou A, Ramani R. 2012. Comparison of the Sensititre YeastOne colorimetric antifungal panel with CLSI microdilution for antifungal susceptibility testing of the echinocandins against Candida spp., using new clinical breakpoints and epidemiological cutoff values. Diagn Microbiol Infect Dis 73:365–368. doi: 10.1016/j.diagmicrobio.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 86.Martín-Mazuelos E, Pemán J, Valverde A, Chaves M, Serrano MC, Cantón E. 2003. Comparison of the Sensititre YeastOne colorimetric antifungal panel and Etest with the NCCLS M38-A method to determine the activity of amphotericin B and itraconazole against clinical isolates of Aspergillus spp. J Antimicrob Chemother 52:365–370. doi: 10.1093/jac/dkg384. [DOI] [PubMed] [Google Scholar]
- 87.Castro C, Serrano MC, Flores B, Espinel-Ingroff A, Martín-Mazuelos E. 2004. Comparison of the Sensititre YeastOne colorimetric antifungal panel with a modified NCCLS M38-A method to determine the activity of voriconazole against clinical isolates of Aspergillus spp. J Clin Microbiol 42:4358–4360. doi: 10.1128/JCM.42.9.4358-4360.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Guinea J, Pelaez T, Alcala L, Bouza E. 2006. Comparison of Sensititre YeastOne with the NCCLS M38-A microdilution method to determine the activity of amphotericin B, voriconazole, and itraconazole against clinical isolates of Aspergillus fumigatus. Diagn Microbiol Infect Dis 56:53–55. doi: 10.1016/j.diagmicrobio.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 89.Patel R, Mendrick C, Knapp CC, Grist R, McNicholas PM. 2007. Clinical evaluation of the Sensititre YeastOne plate for testing susceptibility of filamentous fungi to posaconazole. J Clin Microbiol 45:2000–2001. doi: 10.1128/JCM.00287-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Alvarado-Ramirez E, Torres-Rodriguez JM. 2007. In vitro susceptibility of Sporothrix schenckii to six antifungal agents determined using three different methods. Antimicrob Agents Chemother 51:2420–2423. doi: 10.1128/AAC.01176-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang HC, Hsieh MI, Choi PC, Wu CJ. 2018. Comparison of the Sensititre YeastOne and CLSI M38-A2 microdilution methods in determining the activity of amphotericin B, itraconazole, voriconazole, and posaconazole against Aspergillus species. J Clin Microbiol 56:e00780-18. doi: 10.1128/JCM.00780-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Halliday CL, Chen SC, Kidd SE, van Hal S, Chapman B, Heath CH, Lee A, Kennedy KJ, Daveson K, Sorrell TC, Morrissey CO, Marriott DJ, Slavin MA. 2016. Antifungal susceptibilities of non-Aspergillus filamentous fungi causing invasive infection in Australia: support for current antifungal guideline recommendations. Int J Antimicrob Agents 48:453–458. doi: 10.1016/j.ijantimicag.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 93.Pfaller MA, Diekema DJ, Procop GW, Rinaldi MG. 2007. Multicenter comparison of the VITEK 2 antifungal susceptibility test with the CLSI broth microdilution reference method for testing amphotericin B, flucytosine, and voriconazole against Candida spp. J Clin Microbiol 45:3522–3528. doi: 10.1128/JCM.00403-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Posteraro B, Martucci R, La Sorda M, Fiori B, Sanglard D, De Carolis E, Florio AR, Fadda G, Sanguinetti M. 2009. Reliability of the Vitek 2 yeast susceptibility test for detection of in vitro resistance to fluconazole and voriconazole in clinical isolates of Candida albicans and Candida glabrata. J Clin Microbiol 47:1927–1930. doi: 10.1128/JCM.02070-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bourgeois N, Dehandschoewercker L, Bertout S, Bousquet PJ, Rispail P, Lachaud L. 2010. Antifungal susceptibility of 205 Candida spp. isolated primarily during invasive candidiasis and comparison of the Vitek 2 system with the CLSI broth microdilution and Etest methods. J Clin Microbiol 48:154–161. doi: 10.1128/JCM.01096-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cejudo MA, Gallego AG, Lacasa EC, Aller AI, Romero A, Garcia JP, Andres GQ, Martin-Mazuelos E. 2010. Evaluation of the VITEK 2 system to test the susceptibility of Candida spp., Trichosporon asahii and Cryptococcus neoformans to amphotericin B, flucytosine, fluconazole and voriconazole: a comparison with the M27-A3 reference method. Med Mycol 48:710–719. doi: 10.3109/13693780903473343. [DOI] [PubMed] [Google Scholar]
- 97.Cuenca-Estrella M, Gomez-Lopez A, Alastruey-Izquierdo A, Bernal-Martinez L, Cuesta I, Buitrago MJ, Rodriguez-Tudela JL. 2010. Comparison of the Vitek 2 antifungal susceptibility system with the Clinical and Laboratory Standards Institute (CLSI) and European Committee on Antimicrobial Susceptibility Testing (EUCAST) broth microdilution reference methods and with the Sensititre YeastOne and Etest techniques for in vitro detection of antifungal resistance in yeast isolates. J Clin Microbiol 48:1782–1786. doi: 10.1128/JCM.02316-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Farina C, Manso E, Andreoni S, Conte M, Fazii P, Lombardi G, Sanna S, Russello G. 2011. Interlaboratory evaluation of VITEK2 system and Sensititre YeastOne(R) for antifungal susceptibility testing of yeasts isolated from blood cultures against four antifungal agents. New Microbiol 34:195–201. [PubMed] [Google Scholar]
- 99.Peterson JF, Pfaller MA, Diekema DJ, Rinaldi MG, Riebe KM, Ledeboer NA. 2011. Multicenter comparison of the Vitek 2 antifungal susceptibility test with the CLSI broth microdilution reference method for testing caspofungin, micafungin, and posaconazole against Candida spp. J Clin Microbiol 49:1765–1771. doi: 10.1128/JCM.02517-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Astvad KM, Perlin DS, Johansen HK, Jensen RH, Arendrup MC. 2013. Evaluation of caspofungin susceptibility testing by the new Vitek 2 AST-YS06 yeast card using a unique collection of FKS wild-type and hot spot mutant isolates, including the five most common Candida species. Antimicrob Agents Chemother 57:177–182. doi: 10.1128/AAC.01382-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pfaller MA, Diekema DJ, Procop GW, Rinaldi MG. 2013. Comparison of the Vitek 2 yeast susceptibility system with CLSI microdilution for antifungal susceptibility testing of fluconazole and voriconazole against Candida spp., using new clinical breakpoints and epidemiological cutoff values. Diagn Microbiol Infect Dis 77:37–40. doi: 10.1016/j.diagmicrobio.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 102.Mahabeer Y, Chang CC, Naidu D, Dorasamy A, Lewin S, Ndung'u T, Moosa M-Y, French M, Mlisana K, Coovadia Y. 2014. Comparison of Etests and Vitek 2 (R) to broth microdilution for the susceptibility testing of Cryptococcus neoformans. Diagn Microbiol Infect Dis 80:294–298. doi: 10.1016/j.diagmicrobio.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 103.Pfaller MA, Diekema DJ, Procop GW, Wiederhold NP. 2014. Multicenter evaluation of the new Vitek 2 yeast susceptibility test using new CLSI clinical breakpoints for fluconazole. J Clin Microbiol 52:2126–2130. doi: 10.1128/JCM.00658-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rudensky B, Broidie E, Yinnon AM, Weitzman T, Paz E, Keller N, Raveh D. 2005. Rapid flow-cytometric susceptibility testing of Candida species. J Antimicrob Chemother 55:106–109. doi: 10.1093/jac/dkh492. [DOI] [PubMed] [Google Scholar]
- 105.Wenisch C, Linnau KF, Parschalk B, Zedtwitz-Liebenstein K, Georgopoulos A. 1997. Rapid susceptibility testing of fungi by flow cytometry using vital staining. J Clin Microbiol 35:5–10. doi: 10.1128/JCM.35.1.5-10.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wenisch C, Moore CB, Krause R, Presterl E, Pichna P, Denning DW. 2001. Antifungal susceptibility testing of fluconazole by flow cytometry correlates with clinical outcome. J Clin Microbiol 39:2458–2462. doi: 10.1128/JCM.39.7.2458-2462.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ramani R, Chaturvedi V. 2000. Flow cytometry antifungal susceptibility testing of pathogenic yeasts other than Candida albicans and comparison with the NCCLS broth microdilution test. Antimicrob Agents Chemother 44:2752–2758. doi: 10.1128/aac.44.10.2752-2758.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Peyron F, Favel A, Guiraud-Dauriac H, El Mzibri M, Chastin C, Dumenil G, Regli P. 1997. Evaluation of a flow cytofluorometric method for rapid determination of amphotericin B susceptibility of yeast isolates. Antimicrob Agents Chemother 41:1537–1540. doi: 10.1128/AAC.41.7.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chaturvedi V, Ramani R, Pfaller MA. 2004. Collaborative study of the NCCLS and flow cytometry methods for antifungal susceptibility testing of Candida albicans. J Clin Microbiol 42:2249–2251. doi: 10.1128/jcm.42.5.2249-2251.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pina-Vaz C, Sansonetty F, Rodrigues AG, Costa-Oliveira S, Tavares C, Martinez-de-Oliveira J. 2001. Cytometric approach for a rapid evaluation of susceptibility of Candida strains to antifungals. Clin Microbiol Infect 7:609–618. doi: 10.1046/j.1198-743x.2001.00307.x. [DOI] [PubMed] [Google Scholar]
- 111.Mitchell M, Hudspeth M, Wright A. 2005. Flow cytometry susceptibility testing for the antifungal caspofungin. J Clin Microbiol 43:2586–2589. doi: 10.1128/JCM.43.6.2586-2589.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Vale-Silva LA, Pinto P, Lopes V, Ramos H, Pinto E. 2012. Comparison of the Etest and a rapid flow cytometry-based method with the reference CLSI broth microdilution protocol M27-A3 for the echinocandin susceptibility testing of Candida spp. Eur J Clin Microbiol Infect Dis 31:941–946. doi: 10.1007/s10096-011-1390-z. [DOI] [PubMed] [Google Scholar]
- 113.Wolk DM, Clark AE. 2018. Matrix-assisted laser desorption time of flight mass spectrometry. Clin Lab Med 38:471–486. doi: 10.1016/j.cll.2018.05.008. [DOI] [PubMed] [Google Scholar]
- 114.Sanguinetti M, Posteraro B. 2016. Mass spectrometry applications in microbiology beyond microbe identification: progress and potential. Expert Rev Proteomics 13:965–977. doi: 10.1080/14789450.2016.1231578. [DOI] [PubMed] [Google Scholar]
- 115.Marinach C, Alanio A, Palous M, Kwasek S, Fekkar A, Brossas JY, Brun S, Snounou G, Hennequin C, Sanglard D, Datry A, Golmard JL, Mazier D. 2009. MALDI-TOF MS-based drug susceptibility testing of pathogens: the example of Candida albicans and fluconazole. Proteomics 9:4627–4631. doi: 10.1002/pmic.200900152. [DOI] [PubMed] [Google Scholar]
- 116.De Carolis E, Vella A, Florio AR, Posteraro P, Perlin DS, Sanguinetti M, Posteraro B. 2012. Use of matrix-assisted laser desorption ionization-time of flight mass spectrometry for caspofungin susceptibility testing of Candida and Aspergillus species. J Clin Microbiol 50:2479–2483. doi: 10.1128/JCM.00224-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Vatanshenassan M, Boekhout T, Lass-Florl C, Lackner M, Schubert S, Kostrzewa M, Sparbier K. 2018. Proof of concept for MBT ASTRA, a rapid matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS)-based method to detect caspofungin resistance in Candida albicans and Candida glabrata. J Clin Microbiol 56:e00420-18. doi: 10.1128/JCM.00420-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Saracli MA, Fothergill AW, Sutton DA, Wiederhold NP. 2015. Detection of triazole resistance among Candida species by matrix-assisted laser desorption/ionization-time of flight mass spectrometry (MALDI-TOF MS). Med Mycol 53:736–742. doi: 10.1093/mmy/myv046. [DOI] [PubMed] [Google Scholar]
- 119.Vella A, De Carolis E, Vaccaro L, Posteraro P, Perlin DS, Kostrzewa M, Posteraro B, Sanguinetti M. 2013. Rapid antifungal susceptibility testing by matrix-assisted laser desorption ionization-time of flight mass spectrometry analysis. J Clin Microbiol 51:2964–2969. doi: 10.1128/JCM.00903-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Vella A, De Carolis E, Mello E, Perlin DS, Sanglard D, Sanguinetti M, Posteraro B. 2017. Potential use of MALDI-ToF mass spectrometry for rapid detection of antifungal resistance in the human pathogen Candida glabrata. Sci Rep 7:9099. doi: 10.1038/s41598-017-09329-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gitman MR, McTaggart L, Spinato J, Poopalarajah R, Lister E, Husain S, Kus JV. 2017. Antifungal susceptibility testing of Aspergillus spp. by using a composite correlation index (CCI)-based matrix-assisted laser desorption ionization-time of flight mass spectrometry method appears to not offer benefit over traditional broth microdilution testing. J Clin Microbiol 55:2030–2034. doi: 10.1128/JCM.00254-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chadwick SG, Schuyler JA, Vermitsky JP, Adelson ME, Mordechai E, Gygax SE. 2013. X-Plate Technology: a new method for detecting fluconazole resistance in Candida species. J Med Microbiol 62:720–726. doi: 10.1099/jmm.0.054445-0. [DOI] [PubMed] [Google Scholar]
- 123.de Vasconcelos AA Jr, Menezes EA, Cunha FA. 2011. Chromogenic medium for direct susceptibility testing of Candida spp. isolated from urine. Mycopathologia 172:125–130. doi: 10.1007/s11046-011-9407-9. [DOI] [PubMed] [Google Scholar]
- 124.Kirkpatrick WR, Zimmerman JD, Haikal FP, Broker MJ, Brockway E, Fothergill AW, McCarthy DI, Patterson TF, Redding SW. 2010. Screening for drug-resistant Candida yeasts with chromogenic agar. Med Mycol 48:807–816. doi: 10.3109/13693780903514542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tan GL, Peterson EM. 2005. CHROMagar Candida medium for direct susceptibility testing of yeast from blood cultures. J Clin Microbiol 43:1727–1731. doi: 10.1128/JCM.43.4.1727-1731.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Montesinos I, Argudin MA, Hites M, Ahajjam F, Dodemont M, Dagyaran C, Bakkali M, Etienne I, Jacobs F, Knoop C, Patteet S, Lagrou K. 2017. Culture-based methods and molecular tools for azole-resistant Aspergillus fumigatus detection in a Belgian university hospital. J Clin Microbiol 55:2391–2399. doi: 10.1128/JCM.00520-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bragazzi NL, Gasparini R, Amicizia D, Panatto D, Larosa C. 2015. Porous alumina as a promising biomaterial for public health. Adv Protein Chem Struct Biol 101:213–229. doi: 10.1016/bs.apcsb.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 128.Ingham CJ, Boonstra S, Levels S, de Lange M, Meis JF, Schneeberger PM. 2012. Rapid susceptibility testing and microcolony analysis of Candida spp. cultured and imaged on porous aluminum oxide. PLoS One 7:e33818. doi: 10.1371/journal.pone.0033818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ingham CJ, Sprenkels A, Bomer J, Molenaar D, van den Berg A, van Hylckama Vlieg JE, de Vos WM. 2007. The micro-Petri dish, a million-well growth chip for the culture and high-throughput screening of microorganisms. Proc Natl Acad Sci U S A 104:18217–18222. doi: 10.1073/pnas.0701693104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ingham CJ, Schneeberger PM. 2012. Microcolony imaging of Aspergillus fumigatus treated with echinocandins reveals both fungistatic and fungicidal activities. PLoS One 7:e35478. doi: 10.1371/journal.pone.0035478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ingham CJ, van den Ende M, Wever PC, Schneeberger PM. 2006. Rapid antibiotic sensitivity testing and trimethoprim-mediated filamentation of clinical isolates of the Enterobacteriaceae assayed on a novel porous culture support. J Med Microbiol 55:1511–1519. doi: 10.1099/jmm.0.46585-0. [DOI] [PubMed] [Google Scholar]
- 132.Manneck T, Braissant O, Haggenmuller Y, Keiser J. 2011. Isothermal microcalorimetry to study drugs against Schistosoma mansoni. J Clin Microbiol 49:1217–1225. doi: 10.1128/JCM.02382-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Entenza JM, Betrisey B, Manuel O, Giddey M, Sakwinska O, Laurent F, Bizzini A. 2014. Rapid detection of Staphylococcus aureus strains with reduced susceptibility to vancomycin by isothermal microcalorimetry. J Clin Microbiol 52:180–186. doi: 10.1128/JCM.01820-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Boillat-Blanco N, Furustrand Tafin U, Jaton K, Trampuz A. 2015. Susceptibility testing of Mycobacterium abscessus by isothermal microcalorimetry. Diagn Microbiol Infect Dis 83:139–143. doi: 10.1016/j.diagmicrobio.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 135.Furustrand Tafin U, Clauss M, Hauser PM, Bille J, Meis JF, Trampuz A. 2012. Isothermal microcalorimetry: a novel method for real-time determination of antifungal susceptibility of Aspergillus species. Clin Microbiol Infect 18:E241–E245. doi: 10.1111/j.1469-0691.2012.03854.x. [DOI] [PubMed] [Google Scholar]
- 136.Furustrand Tafin U, Meis JF, Trampuz A. 2013. Microcalorimetry assay for rapid detection of voriconazole resistance in Aspergillus fumigatus. Antimicrob Agents Chemother 57:5704–5706. doi: 10.1128/AAC.01379-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Furustrand Tafin U, Meis JF, Trampuz A. 2012. Isothermal microcalorimetry for antifungal susceptibility testing of Mucorales, Fusarium spp., and Scedosporium spp. Diagn Microbiol Infect Dis 73:330–337. doi: 10.1016/j.diagmicrobio.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 138.Maiolo EM, Furustrand Tafin U, Borens O, Trampuz A. 2014. Activities of fluconazole, caspofungin, anidulafungin, and amphotericin B on planktonic and biofilm Candida species determined by microcalorimetry. Antimicrob Agents Chemother 58:2709–2717. doi: 10.1128/AAC.00057-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Rex JH, Pfaller MA. 2002. Has antifungal susceptibility testing come of age? Clin Infect Dis 35:982–989. doi: 10.1086/342384. [DOI] [PubMed] [Google Scholar]
- 140.Pfaller MA, Messer SA, Coffmann S. 1995. Comparison of visual and spectrophotometric methods of MIC endpoint determinations by using broth microdilution methods to test five antifungal agents, including the new triazole D0870. J Clin Microbiol 33:1094–1097. doi: 10.1128/JCM.33.5.1094-1097.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Pfaller MA, Messer SA, Bolmstrom A, Odds FC, Rex JH. 1996. Multisite reproducibility of the Etest MIC method for antifungal susceptibility testing of yeast isolates. J Clin Microbiol 34:1691–1693. doi: 10.1128/JCM.34.7.1691-1693.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Revankar SG, Kirkpatrick WR, McAtee RK, Fothergill AW, Redding SW, Rinaldi MG, Patterson TF. 1998. Interpretation of trailing endpoints in antifungal susceptibility testing by the National Committee for Clinical Laboratory Standards method. J Clin Microbiol 36:153–156. doi: 10.1128/JCM.36.1.153-156.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Marr KA, Rustad TR, Rex JH, White TC. 1999. The trailing end point phenotype in antifungal susceptibility testing is pH dependent. Antimicrob Agents Chemother 43:1383–1386. doi: 10.1128/AAC.43.6.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Arthington-Skaggs BA, Warnock DW, Morrison CJ. 2000. Quantitation of Candida albicans ergosterol content improves the correlation between in vitro antifungal susceptibility test results and in vivo outcome after fluconazole treatment in a murine model of invasive candidiasis. Antimicrob Agents Chemother 44:2081–2085. doi: 10.1128/aac.44.8.2081-2085.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Arthington-Skaggs BA, Candidemia Active Surveillance Group, Lee-Yang W, Ciblak MA, Frade JP, Brandt ME, Hajjeh RA, Harrison LH, Sofair AN, Warnock DW. 2002. Comparison of visual and spectrophotometric methods of broth microdilution MIC end point determination and evaluation of a sterol quantitation method for in vitro susceptibility testing of fluconazole and itraconazole against trailing and nontrailing Candida isolates. Antimicrob Agents Chemother 46:2477–2481. doi: 10.1128/aac.46.8.2477-2481.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Odds FC, Motyl M, Andrade R, Bille J, Canton E, Cuenca-Estrella M, Davidson A, Durussel C, Ellis D, Foraker E, Fothergill AW, Ghannoum MA, Giacobbe RA, Gobernado M, Handke R, Laverdiere M, Lee-Yang W, Merz WG, Ostrosky-Zeichner L, Peman J, Perea S, Perfect JR, Pfaller MA, Proia L, Rex JH, Rinaldi MG, Rodriguez-Tudela JL, Schell WA, Shields C, Sutton DA, Verweij PE, Warnock DW. 2004. Interlaboratory comparison of results of susceptibility testing with caspofungin against Candida and Aspergillus species. J Clin Microbiol 42:3475–3482. doi: 10.1128/JCM.42.8.3475-3482.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Fleischhacker M, Radecke C, Schulz B, Ruhnke M. 2008. Paradoxical growth effects of the echinocandins caspofungin and micafungin, but not of anidulafungin, on clinical isolates of Candida albicans and C. dubliniensis. Eur J Clin Microbiol Infect Dis 27:127–131. doi: 10.1007/s10096-007-0411-4. [DOI] [PubMed] [Google Scholar]
- 148.Chamilos G, Lewis RE, Albert N, Kontoyiannis DP. 2007. Paradoxical effect of echinocandins across Candida species in vitro: evidence for echinocandin-specific and candida species-related differences. Antimicrob Agents Chemother 51:2257–2259. doi: 10.1128/AAC.00095-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Soczo G, Kardos G, Varga I, Kelentey B, Gesztelyi R, Majoros L. 2007. In vitro study of Candida tropicalis isolates exhibiting paradoxical growth in the presence of high concentrations of caspofungin. Antimicrob Agents Chemother 51:4474–4476. doi: 10.1128/AAC.00880-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Stevens DA, Espiritu M, Parmar R. 2004. Paradoxical effect of caspofungin: reduced activity against Candida albicans at high drug concentrations. Antimicrob Agents Chemother 48:3407–3411. doi: 10.1128/AAC.48.9.3407-3411.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Stevens DA, White TC, Perlin DS, Selitrennikoff CP. 2005. Studies of the paradoxical effect of caspofungin at high drug concentrations. Diagn Microbiol Infect Dis 51:173–178. doi: 10.1016/j.diagmicrobio.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 152.Eagle H, Musselman AD. 1948. The rate of bactericidal action of penicillin in vitro as a function of its concentration, and its paradoxically reduced activity at high concentrations against certain organisms. J Exp Med 88:99–131. doi: 10.1084/jem.88.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Stevens DA, Ichinomiya M, Koshi Y, Horiuchi H. 2006. Escape of Candida from caspofungin inhibition at concentrations above the MIC (paradoxical effect) accomplished by increased cell wall chitin; evidence for beta-1,6-glucan synthesis inhibition by caspofungin. Antimicrob Agents Chemother 50:3160–3161. doi: 10.1128/AAC.00563-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Clemons KV, Espiritu M, Parmar R, Stevens DA. 2006. Assessment of the paradoxical effect of caspofungin in therapy of candidiasis. Antimicrob Agents Chemother 50:1293–1297. doi: 10.1128/AAC.50.4.1293-1297.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Pfaller MA, Andes D, Arendrup MC, Diekema DJ, Espinel-Ingroff A, Alexander BD, Brown SD, Chaturvedi V, Fowler CL, Ghannoum MA, Johnson EM, Knapp CC, Motyl MR, Ostrosky-Zeichner L, Walsh TJ. 2011. Clinical breakpoints for voriconazole and Candida spp. revisited: review of microbiologic, molecular, pharmacodynamic, and clinical data as they pertain to the development of species-specific interpretive criteria. Diagn Microbiol Infect Dis 70:330–343. doi: 10.1016/j.diagmicrobio.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 156.Pfaller MA, CLSI Subcommittee for Antifungal Testing, Diekema DJ, Andes D, Arendrup MC, Brown SD, Lockhart SR, Motyl M, Perlin DS. 2011. Clinical breakpoints for the echinocandins and Candida revisited: integration of molecular, clinical, and microbiological data to arrive at species-specific interpretive criteria. Drug Resist Updat 14:164–176. doi: 10.1016/j.drup.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 157.Pfaller MA, CLSI Subcommittee for Antifungal Susceptibility Testing, Andes D, Diekema DJ, Espinel-Ingroff A, Sheehan D. 2010. Wild-type MIC distributions, epidemiological cutoff values and species-specific clinical breakpoints for fluconazole and Candida: time for harmonization of CLSI and EUCAST broth microdilution methods. Drug Resist Updat 13:180–195. doi: 10.1016/j.drup.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 158.European Committee on Antimicrobial Susceptibility Testing (EUCAST). 2018. Antifungal agents: breakpoint tables for interpretation of MICs, version 9.0. http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/AFST/Clinical_breakpoints/Antifungal_breakpoints_v_9.0_180212.pdf.
- 159.Arendrup MC, European Committee on Antimicrobial Susceptibility Testing-Subcommittee on Antifungal Susceptibility Testing (EUCAST-AFST), Cuenca-Estrella M, Lass-Flörl C, Hope WW. 2014. EUCAST technical note on Candida and micafungin, anidulafungin and fluconazole. Mycoses 57:377–379. doi: 10.1111/myc.12170. [DOI] [PubMed] [Google Scholar]
- 160.Arendrup MC, European Committee on Antimicrobial Susceptibility Testing-Subcommittee on Antifungal Susceptibility Testing (EUCAST-AFST), Cuenca-Estrella M, Donnelly JP, Hope W, Lass-Flörl C, Rodriguez-Tudela J-L. 2011. EUCAST technical note on posaconazole. Clin Microbiol Infect 17:E16–E17. doi: 10.1111/j.1469-0691.2011.03646.x. [DOI] [PubMed] [Google Scholar]
- 161.Lass-Florl C, European Committee on Antimicrobial Susceptibility Testing-Subcommittee on Antifungal Susceptibility Testing, Arendrup MC, Rodriguez-Tudela JL, Cuenca-Estrella M, Donnelly P, Hope W. 2011. EUCAST technical note on amphotericin B. Clin Microbiol Infect 17:E27–E29. doi: 10.1111/j.1469-0691.2011.03644.x. [DOI] [PubMed] [Google Scholar]
- 162.European Committee on Antimicrobial Susceptibility Testing (EUCAST). 17 November 2017. Standard operating procedure: MIC distributions and the setting of epidemiological cut-off (ECOFF) values. http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/EUCAST_SOPs/EUCAST_SOP_10.0_MIC_distributions_and_epidemiological_cut-off_value__ECOFF__setting_20171117.pdf.
- 163.Clinical and Laboratory Standards Institute. 2016. Principles and procedures for the development of epidemiological cutoff values for antifungal susceptibility testing, 1st ed Guideline M57 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 164.Clinical and Laboratory Standards Institute. 2018. Epidemiological cutoff values for antifungal susceptibility testing, 2nd ed Supplement M59 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 165.Alexander BD, Johnson MD, Pfeiffer CD, Jimenez-Ortigosa C, Catania J, Booker R, Castanheira M, Messer SA, Perlin DS, Pfaller MA. 2013. Increasing echinocandin resistance in Candida glabrata: clinical failure correlates with presence of FKS mutations and elevated minimum inhibitory concentrations. Clin Infect Dis 56:1724–1732. doi: 10.1093/cid/cit136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Shields RK, Nguyen MH, Press EG, Kwa AL, Cheng S, Du C, Clancy CJ. 2012. The presence of an FKS mutation rather than MIC is an independent risk factor for failure of echinocandin therapy among patients with invasive candidiasis due to Candida glabrata. Antimicrob Agents Chemother 56:4862–4869. doi: 10.1128/AAC.00027-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Lockhart SR, Ghannoum MA, Alexander BD. 2017. Establishment and use of epidemiological cutoff values for molds and yeasts by use of the Clinical and Laboratory Standards Institute M57 standard. J Clin Microbiol 55:1262–1268. doi: 10.1128/JCM.02416-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Odds FC, Brown AJ, Gow NA. 2003. Antifungal agents: mechanisms of action. Trends Microbiol 11:272–279. doi: 10.1016/s0966-842x(03)00117-3. [DOI] [PubMed] [Google Scholar]
- 169.Perlin DS, Rautemaa-Richardson R, Alastruey-Izquierdo A. 2017. The global problem of antifungal resistance: prevalence, mechanisms, and management. Lancet Infect Dis 17:e383–e392. doi: 10.1016/S1473-3099(17)30316-X. [DOI] [PubMed] [Google Scholar]
- 170.Lockhart SR, Iqbal N, Cleveland AA, Farley MM, Harrison LH, Bolden CB, Baughman W, Stein B, Hollick R, Park BJ, Chiller T. 2012. Species identification and antifungal susceptibility testing of Candida bloodstream isolates from population-based surveillance studies in two U.S. cities from 2008 to 2011. J Clin Microbiol 50:3435–3442. doi: 10.1128/JCM.01283-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Hesstvedt L, Norwegian Yeast Study Group, Gaustad P, Andersen CT, Haarr E, Hannula R, Haukland HH, Hermansen NO, Larssen KW, Mylvaganam H, Ranheim TE, Sandven P, Nordoy I, Norwegian Yeast Study G, Kanestrom A, Grub C, Onken A, Thielsen C, Skaare D, Tofteland S, Sonsteby LJ, Hjetland R, Hide R, Vik E, Kummel A, Asheim S. 2015. Twenty-two years of candidaemia surveillance: results from a Norwegian national study. Clin Microbiol Infect 21:938–945. doi: 10.1016/j.cmi.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 172.Orasch C, Marchetti O, Garbino J, Schrenzel J, Zimmerli S, Muhlethaler K, Pfyffer G, Ruef C, Fehr J, Zbinden R, Calandra T, Bille J, Funginos . 2014. Candida species distribution and antifungal susceptibility testing according to European Committee on Antimicrobial Susceptibility Testing and new vs. old Clinical and Laboratory Standards Institute clinical breakpoints: a 6-year prospective candidaemia survey from the fungal infection network of Switzerland. Clin Microbiol Infect 20:698–705. doi: 10.1111/1469-0691.12440. [DOI] [PubMed] [Google Scholar]
- 173.Tan TY, Hsu LY, Alejandria MM, Chaiwarith R, Chinniah T, Chayakulkeeree M, Choudhury S, Chen YH, Shin JH, Kiratisin P, Mendoza M, Prabhu K, Supparatpinyo K, Tan AL, Phan XT, Tran TT, Nguyen GB, Doan MP, Huynh VA, Nguyen SM, Tran TB, Van Pham H. 2016. Antifungal susceptibility of invasive Candida bloodstream isolates from the Asia-Pacific region. Med Mycol 54:471–477. doi: 10.1093/mmy/myv114. [DOI] [PubMed] [Google Scholar]
- 174.Chakrabarti A, Sood P, Rudramurthy SM, Chen S, Kaur H, Capoor M, Chhina D, Rao R, Eshwara VK, Xess I, Kindo AJ, Umabala P, Savio J, Patel A, Ray U, Mohan S, Iyer R, Chander J, Arora A, Sardana R, Roy I, Appalaraju B, Sharma A, Shetty A, Khanna N, Marak R, Biswas S, Das S, Harish BN, Joshi S, Mendiratta D. 2015. Incidence, characteristics and outcome of ICU-acquired candidemia in India. Intensive Care Med 41:285–295. doi: 10.1007/s00134-014-3603-2. [DOI] [PubMed] [Google Scholar]
- 175.Chapman B, Slavin M, Marriott D, Halliday C, Kidd S, Arthur I, Bak N, Heath CH, Kennedy K, Morrissey CO, Sorrell TC, van Hal S, Keighley C, Goeman E, Underwood N, Hajkowicz K, Hofmeyr A, Leung M, Macesic N, Botes J, Blyth C, Cooley L, George CR, Kalukottege P, Kesson A, McMullan B, Baird R, Robson J, Korman TM, Pendle S, Weeks K, Liu E, Cheong E, Chen S, Australian and New Zealand Mycoses Interest Group . 2017. Changing epidemiology of candidaemia in Australia. J Antimicrob Chemother 72:1103–1108. doi: 10.1093/jac/dkw422. [DOI] [PubMed] [Google Scholar]
- 176.Hii IM, Chang HL, Lin LC, Lee YL, Liu YM, Liu CE, Chen CH, Cheng YR, Chang CY. 2015. Changing epidemiology of candidemia in a medical center in middle Taiwan. J Microbiol Immunol Infect 48:306–315. doi: 10.1016/j.jmii.2013.08.017. [DOI] [PubMed] [Google Scholar]
- 177.Trouve C, Blot S, Hayette MP, Jonckheere S, Patteet S, Rodriguez-Villalobos H, Symoens F, Van Wijngaerden E, Lagrou K. 2017. Epidemiology and reporting of candidaemia in Belgium: a multi-centre study. Eur J Clin Microbiol Infect Dis 36:649–655. doi: 10.1007/s10096-016-2841-3. [DOI] [PubMed] [Google Scholar]
- 178.Castanheira M, Deshpande LM, Davis AP, Rhomberg PR, Pfaller MA. 2017. Monitoring antifungal resistance in a global collection of invasive yeasts and molds: application of CLSI epidemiological cutoff values and whole-genome sequencing analysis for detection of azole resistance in Candida albicans. Antimicrob Agents Chemother 61:e00906-17. doi: 10.1128/AAC.00906-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Vallabhaneni S, Cleveland AA, Farley MM, Harrison LH, Schaffner W, Beldavs ZG, Derado G, Pham CD, Lockhart SR, Smith RM. 2015. Epidemiology and risk factors for echinocandin nonsusceptible Candida glabrata bloodstream infections: data from a large multisite population-based candidemia surveillance program, 2008–2014. Open Forum Infect Dis 2:ofv163. doi: 10.1093/ofid/ofv163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Pfaller MA, Castanheira M, Lockhart SR, Ahlquist AM, Messer SA, Jones RN. 2012. Frequency of decreased susceptibility and resistance to echinocandins among fluconazole-resistant bloodstream isolates of Candida glabrata. J Clin Microbiol 50:1199–1203. doi: 10.1128/JCM.06112-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Klotz U, Schmidt D, Willinger B, Steinmann E, Buer J, Rath PM, Steinmann J. 2016. Echinocandin resistance and population structure of invasive Candida glabrata isolates from two university hospitals in Germany and Austria. Mycoses 59:312–318. doi: 10.1111/myc.12472. [DOI] [PubMed] [Google Scholar]
- 182.Lepak AJ, Zhao M, Berkow EL, Lockhart SR, Andes DR. 2017. Pharmacodynamic optimization for treatment of invasive Candida auris infection. Antimicrob Agents Chemother 61:e00791-17. doi: 10.1128/AAC.00791-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Verweij PE, Chowdhary A, Melchers WJ, Meis JF. 2016. Azole resistance in Aspergillus fumigatus: can we retain the clinical use of mold-active antifungal azoles? Clin Infect Dis 62:362–368. doi: 10.1093/cid/civ885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Verweij PE, Mellado E, Melchers WJ. 2007. Multiple-triazole-resistant aspergillosis. N Engl J Med 356:1481–1483. doi: 10.1056/NEJMc061720. [DOI] [PubMed] [Google Scholar]
- 185.Verweij PE, Snelders E, Kema GH, Mellado E, Melchers WJ. 2009. Azole resistance in Aspergillus fumigatus: a side-effect of environmental fungicide use? Lancet Infect Dis 9:789–795. doi: 10.1016/S1473-3099(09)70265-8. [DOI] [PubMed] [Google Scholar]
- 186.Chowdhary A, Kathuria S, Xu J, Meis JF. 2013. Emergence of azole-resistant aspergillus fumigatus strains due to agricultural azole use creates an increasing threat to human health. PLoS Pathog 9:e1003633. doi: 10.1371/journal.ppat.1003633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Bader O, Tünnermann J, Dudakova A, Tangwattanachuleeporn M, Weig M, Groß U. 2015. Environmental isolates of azole-resistant Aspergillus fumigatus in Germany. Antimicrob Agents Chemother 59:4356–4359. doi: 10.1128/AAC.00100-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Chowdhary A, Sharma C, van den Boom M, Yntema JB, Hagen F, Verweij PE, Meis JF. 2014. Multi-azole-resistant Aspergillus fumigatus in the environment in Tanzania. J Antimicrob Chemother 69:2979–2983. doi: 10.1093/jac/dku259. [DOI] [PubMed] [Google Scholar]
- 189.Chowdhary A, Sharma C, Kathuria S, Hagen F, Meis JF. 2015. Prevalence and mechanism of triazole resistance in Aspergillus fumigatus in a referral chest hospital in Delhi, India and an update of the situation in Asia. Front Microbiol 6:428. doi: 10.3389/fmicb.2015.00428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Kidd SE, Goeman E, Meis JF, Slavin MA, Verweij PE. 2015. Multi-triazole-resistant Aspergillus fumigatus infections in Australia. Mycoses 58:350–355. doi: 10.1111/myc.12324. [DOI] [PubMed] [Google Scholar]
- 191.Lavergne RA, Morio F, Favennec L, Dominique S, Meis JF, Gargala G, Verweij PE, Le Pape P. 2015. First description of azole-resistant Aspergillus fumigatus due to TR46/Y121F/T289A mutation in France. Antimicrob Agents Chemother 59:4331–4335. doi: 10.1128/AAC.00127-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Le Pape P, Lavergne RA, Morio F, Alvarez-Moreno C. 2016. Multiple fungicide-driven alterations in azole-resistant Aspergillus fumigatus, Colombia, 2015. Emerg Infect Dis 22:156–157. doi: 10.3201/eid2201.150978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Lockhart SR, Frade JP, Etienne KA, Pfaller MA, Diekema DJ, Balajee SA. 2011. Azole resistance in Aspergillus fumigatus isolates from the ARTEMIS global surveillance study is primarily due to the TR/L98H mutation in the cyp51A gene. Antimicrob Agents Chemother 55:4465–4468. doi: 10.1128/AAC.00185-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Vermeulen E, Maertens J, De Bel A, Nulens E, Boelens J, Surmont I, Mertens A, Boel A, Lagrou K. 2015. Nationwide surveillance of azole resistance in aspergillus diseases. Antimicrob Agents Chemother 59:4569–4576. doi: 10.1128/AAC.00233-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Mylonakis E, Clancy CJ, Ostrosky-Zeichner L, Garey KW, Alangaden GJ, Vazquez JA, Groeger JS, Judson MA, Vinagre YM, Heard SO, Zervou FN, Zacharioudakis IM, Kontoyiannis DP, Pappas PG. 2015. T2 magnetic resonance assay for the rapid diagnosis of candidemia in whole blood: a clinical trial. Clin Infect Dis 60:892–899. doi: 10.1093/cid/ciu959. [DOI] [PubMed] [Google Scholar]
- 196.Wallet F, Nseir S, Baumann L, Herwegh S, Sendid B, Boulo M, Roussel-Delvallez M, Durocher AV, Courcol RJ. 2010. Preliminary clinical study using a multiplex real-time PCR test for the detection of bacterial and fungal DNA directly in blood. Clin Microbiol Infect 16:774–779. doi: 10.1111/j.1469-0691.2009.02940.x. [DOI] [PubMed] [Google Scholar]
- 197.Chong GL, van de Sande WW, Dingemans GJ, Gaajetaan GR, Vonk AG, Hayette MP, van Tegelen DW, Simons GF, Rijnders BJ. 2015. Validation of a new Aspergillus real-time PCR assay for direct detection of Aspergillus and azole resistance of Aspergillus fumigatus on bronchoalveolar lavage fluid. J Clin Microbiol 53:868–874. doi: 10.1128/JCM.03216-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Dannaoui E, Gabriel F, Gaboyard M, Lagardere G, Audebert L, Quesne G, Godichaud S, Verweij PE, Accoceberry I, Bougnoux ME. 2017. Molecular diagnosis of invasive aspergillosis and detection of azole resistance by a newly commercialized PCR kit. J Clin Microbiol 55:3210–3218. doi: 10.1128/JCM.01032-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Perlin DS. 2007. Resistance to echinocandin-class antifungal drugs. Drug Resist Updat 10:121–130. doi: 10.1016/j.drup.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Park S, Kelly R, Kahn JN, Robles J, Hsu MJ, Register E, Li W, Vyas V, Fan H, Abruzzo G, Flattery A, Gill C, Chrebet G, Parent SA, Kurtz M, Teppler H, Douglas CM, Perlin DS. 2005. Specific substitutions in the echinocandin target Fks1p account for reduced susceptibility of rare laboratory and clinical Candida sp. isolates. Antimicrob Agents Chemother 49:3264–3273. doi: 10.1128/AAC.49.8.3264-3273.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Douglas CM, D'Ippolito JA, Shei GJ, Meinz M, Onishi J, Marrinan JA, Li W, Abruzzo GK, Flattery A, Bartizal K, Mitchell A, Kurtz MB. 1997. Identification of the FKS1 gene of Candida albicans as the essential target of 1,3-beta-d-glucan synthase inhibitors. Antimicrob Agents Chemother 41:2471–2479. doi: 10.1128/AAC.41.11.2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Balashov SV, Park S, Perlin DS. 2006. Assessing resistance to the echinocandin antifungal drug caspofungin in Candida albicans by profiling mutations in FKS1. Antimicrob Agents Chemother 50:2058–2063. doi: 10.1128/AAC.01653-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Pham CD, Bolden CB, Kuykendall RJ, Lockhart SR. 2014. Development of a Luminex-based multiplex assay for detection of mutations conferring resistance to echinocandins in Candida glabrata. J Clin Microbiol 52:790–795. doi: 10.1128/JCM.03378-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Zhao Y, Nagasaki Y, Kordalewska M, Press EG, Shields RK, Nguyen MH, Clancy CJ, Perlin DS. 2016. Rapid detection of FKS-associated echinocandin resistance in Candida glabrata. Antimicrob Agents Chemother 60:6573–6577. doi: 10.1128/AAC.01574-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Berkow EL, Lockhart SR. 2017. Fluconazole resistance in Candida species: a current perspective. Infect Drug Resist 10:237–245. doi: 10.2147/IDR.S118892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Cowen LE, Sanglard D, Howard SJ, Rogers PD, Perlin DS. 2014. Mechanisms of antifungal drug resistance. Cold Spring Harb Perspect Med 5:a019752. doi: 10.1101/cshperspect.a019752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.van der Linden JW, Snelders E, Arends JP, Daenen SM, Melchers WJ, Verweij PE. 2010. Rapid diagnosis of azole-resistant aspergillosis by direct PCR using tissue specimens. J Clin Microbiol 48:1478–1480. doi: 10.1128/JCM.02221-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Denning DW, Park S, Lass-Florl C, Fraczek MG, Kirwan M, Gore R, Smith J, Bueid A, Moore CB, Bowyer P, Perlin DS. 2011. High-frequency triazole resistance found in nonculturable Aspergillus fumigatus from lungs of patients with chronic fungal disease. Clin Infect Dis 52:1123–1129. doi: 10.1093/cid/cir179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Zhao Y, Stensvold CR, Perlin DS, Arendrup MC. 2013. Azole resistance in Aspergillus fumigatus from bronchoalveolar lavage fluid samples of patients with chronic diseases. J Antimicrob Chemother 68:1497–1504. doi: 10.1093/jac/dkt071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Buil JB, Zoll J, Verweij PE, Melchers W. 2018. Molecular detection of azole-resistant Aspergillus fumigatus in clinical samples. Front Microbiol 9:515. doi: 10.3389/fmicb.2018.00515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Lestrade PP, Meis JF, Arends JP, van der Beek MT, de Brauwer E, van Dijk K, de Greeff SC, Haas PJ, Hodiamont CJ, Kuijper EJ, Leenstra T, Muller AE, Oude Lashof AM, Rijnders BJ, Roelofsen E, Rozemeijer W, Tersmette M, Terveer EM, Verduin CM, Wolfhagen MJ, Melchers WJ, Verweij PE. 2016. Diagnosis and management of aspergillosis in the Netherlands: a national survey. Mycoses 59:101–107. doi: 10.1111/myc.12440. [DOI] [PubMed] [Google Scholar]
- 212.Chowdhary A, Sharma C, Meis JF. 2017. Azole-resistant aspergillosis: epidemiology, molecular mechanisms, and treatment. J Infect Dis 216:S436–S444. doi: 10.1093/infdis/jix210. [DOI] [PubMed] [Google Scholar]
- 213.Patterson TF, Thompson GR III, Denning DW, Fishman JA, Hadley S, Herbrecht R, Kontoyiannis DP, Marr KA, Morrison VA, Nguyen MH, Segal BH, Steinbach WJ, Stevens DA, Walsh TJ, Wingard JR, Young JA, Bennett JE. 2016. Executive summary: practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis 63:433–442. doi: 10.1093/cid/ciw444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Perfect JR. 2017. The antifungal pipeline: a reality check. Nat Rev Drug Discov 16:603–616. doi: 10.1038/nrd.2017.46. [DOI] [PMC free article] [PubMed] [Google Scholar]