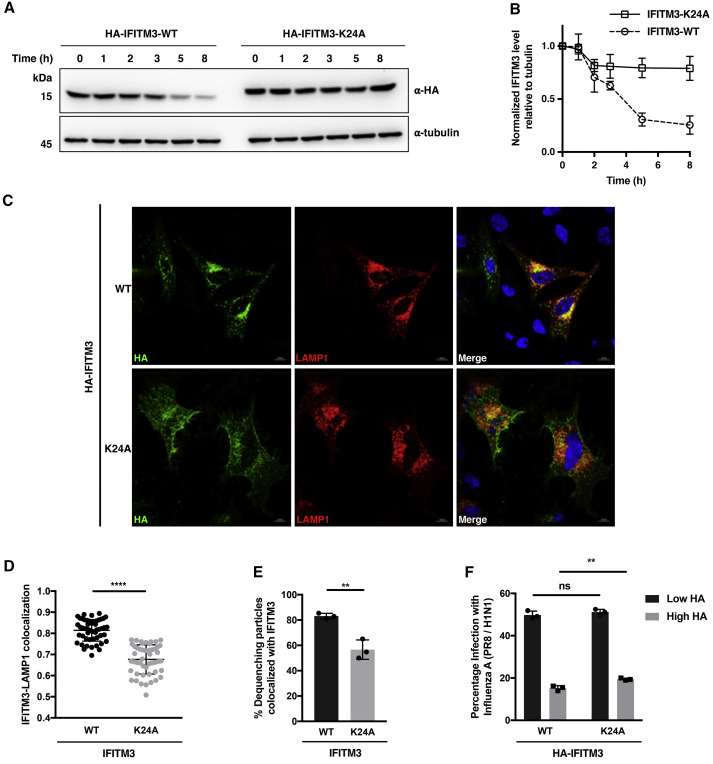
Figure 5.
K24 Ubiquitination Regulates IFITM3 Turnover, Localization, and Co-trafficking with Incoming Virus Particles
(A) Analysis of turnover of IFITM3 and K24A mutant. HeLa cells expressing HA-IFITM3 or K24A mutant were treated with CHX (25 μg/mL) for the indicated times and lysed for anti-HA western blot analysis.
(B) Quantification of IFITM3 levels normalized to tubulin levels shown in (A). Data are represented as mean ± SD, n = 3.
(C) Immunofluorescence analysis of IFITM3 and K24A mutant. HeLa cells were transfected with indicated HA-IFITM3 construct and mCherry-LAMP1, and processed for immunofluorescence with an Alexa Fluor 488-conjugated anti-HA antibody. Scale bars, 10 μm.
(D) Quantification of Pearson coefficients for the IFITM3-LAMP1 colocalization shown in (C). Data are represented as mean ± SD, n = 50 cells. ∗∗∗∗p < 0.0001 calculated by Student's t test.
(E) Relative percentage of DiD-IAV particles colocalized with IFITM3 and IFITM3-K24A at the time of dequenching. HeLa IFITM2/3-KO cells expressing BODIPY-labeled IFITM3 were infected with DiD-IAV particles and monitored for DiD dequenching and IFITM3 trafficking by time-lapse imaging. Data are represented as mean ± SD of three independent experiments. ∗∗p < 0.01 calculated by Student's t test.
(F) Antiviral activity of IFITM3 and K24A mutant. HEK293T cells expressing HA-IFITM3 or HA-IFITM3-K24A were infected with IAV (PR8/H1N1) at an MOI of 2.5 for 6 h. Cells were fixed and stained with anti-HA and anti-influenza NP antibodies to measure IFITM3 expression and virus infection, respectively, with flow cytometry. “Low HA” and “High HA” indicate cell populations expressing low and high levels of HA-IFITM3, respectively. Data are represented as mean ± SD of three independent experiments. ∗∗p < 0.01, n.s. indicates p > 0.05 calculated by Student's t test.