Figure 1. Rem inhibition of reconstituted CaV1.2 channels.
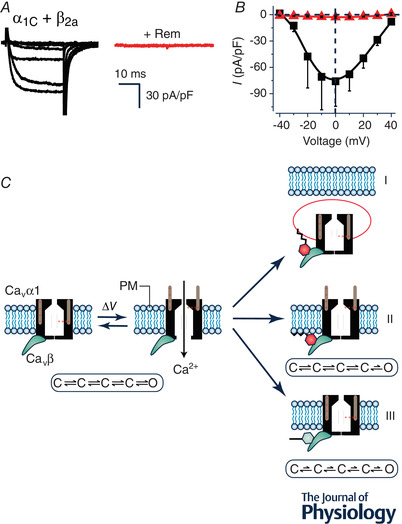
A, exemplar family of whole‐cell Ba2+ currents from recombinant CaV1.2 channels (α1C + β2a) reconstituted in HEK293 cells either without (left) or with (right) co‐expression of Rem. B, population I‐V curves from CaV1.2 channels in the absence (■) or presence ( ) of co‐expressed Rem. C, schematic diagram showing three distinct mechanisms (I‐III) utilized by Rem to inhibit recombinant CaV1.2 channels. In mechanism I, co‐expressed Rem results in a decrease in the number of channels at the cell surface (N) due to enhanced CaV1.2 endocytosis. Mechanism II involves a reduction in the open probability (P
o) of channels residing on the plasma membrane without impacting on voltage sensor movement as measured by total gating charge (Q
max). This mechanism requires Rem simultaneously binding to the CaVβ subunit (using the guanine nucleotide binding domain) and the plasma membrane (via the polybasic distal C‐terminus). Mechanism III involves an impaired movement of the voltage sensor movement of surface channels as measured by a decreased Q
max (observed even when N is completely rescued by co‐expressing dominant negative dynamin). Mechanism III is blocked by a mutation (T94N) that favours GDP over GTP binding to Rem, suggesting it requires GTP‐bound Rem.
) of co‐expressed Rem. C, schematic diagram showing three distinct mechanisms (I‐III) utilized by Rem to inhibit recombinant CaV1.2 channels. In mechanism I, co‐expressed Rem results in a decrease in the number of channels at the cell surface (N) due to enhanced CaV1.2 endocytosis. Mechanism II involves a reduction in the open probability (P
o) of channels residing on the plasma membrane without impacting on voltage sensor movement as measured by total gating charge (Q
max). This mechanism requires Rem simultaneously binding to the CaVβ subunit (using the guanine nucleotide binding domain) and the plasma membrane (via the polybasic distal C‐terminus). Mechanism III involves an impaired movement of the voltage sensor movement of surface channels as measured by a decreased Q
max (observed even when N is completely rescued by co‐expressing dominant negative dynamin). Mechanism III is blocked by a mutation (T94N) that favours GDP over GTP binding to Rem, suggesting it requires GTP‐bound Rem.
