Figure 3. Mimicking RGK‐mediated CaV inhibition mechanisms with an engineered CaVβ‐targeted nanobody.
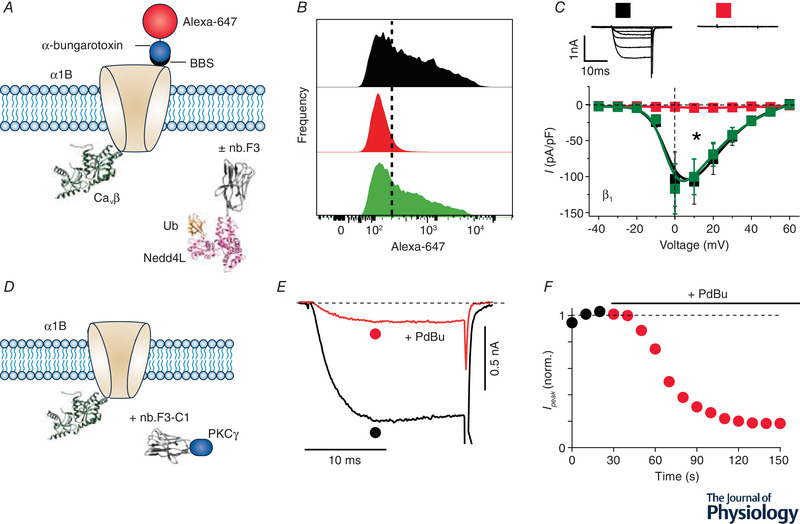
A, schematic diagram of experimental paradigm. Recombinant CaV2.2 (α1B) with an extracellular bungarotoxin‐binding site (BBS) epitope is co‐expressed CaVβ without (control) or with a CaVβ‐targeting nanobody (nb.F3) fused to catalytic HECT domain of the E3 ubiquitin ligase, NEDD4L. Surface channels are measured by exposing non‐permeabilized transfected cells to Alexa 647‐conjugated bungarotoxin. B, histograms of surface BBS‐α1B assessed by flow cytometry in cells expressing no nanobody (top), nb.F3‐NEDD4L (middle), or nb.F3‐NEDD4L*, a catalytically dead variant (bottom). Results show a substantial decline in surface density when the channel is co‐expressed with nb.F3‐NEDD4L. C, exemplar (top) and population I‐V curves (bottom) in cells expressing α1B + β1b alone (■) or with either nb.F3‐NEDD4L ( ) or nb.F3‐NEDD4L* (
) or nb.F3‐NEDD4L* ( ) co‐expression. D, schematic diagram of experimental paradigm. HEK293 cells are co‐transfected with recombinant CaV2.2 (α1B + β3) and nb.F3‐C1PKCγ. E and F, exemplar currents and diary plot showing rapid and deep PdBu‐induced inhibition of CaV2.2 currents in cells expressing α1B + β3 + nb.F3‐C1PKCγ.
) co‐expression. D, schematic diagram of experimental paradigm. HEK293 cells are co‐transfected with recombinant CaV2.2 (α1B + β3) and nb.F3‐C1PKCγ. E and F, exemplar currents and diary plot showing rapid and deep PdBu‐induced inhibition of CaV2.2 currents in cells expressing α1B + β3 + nb.F3‐C1PKCγ.
