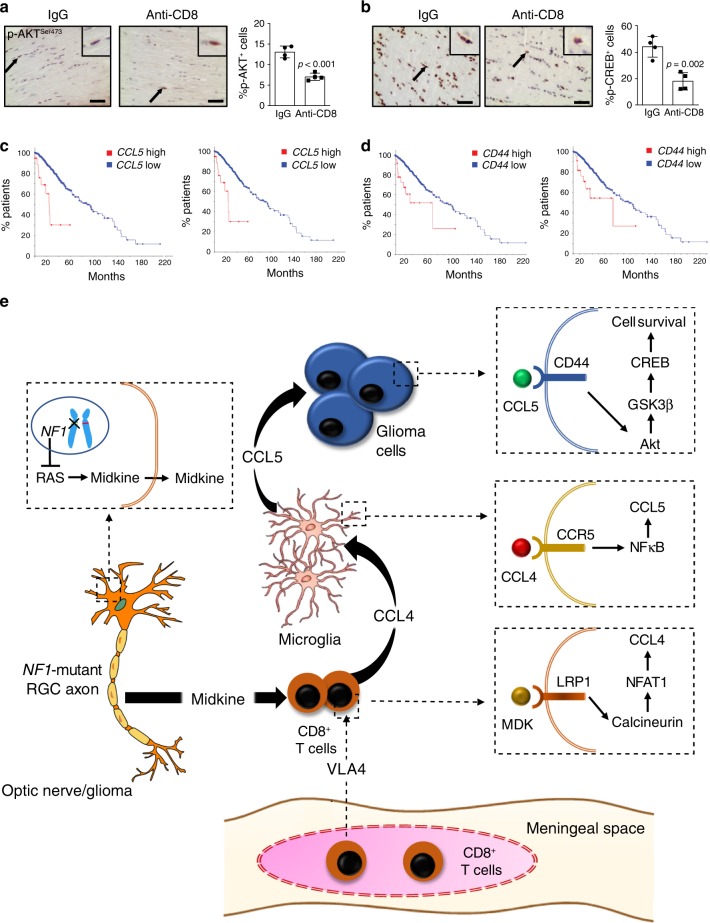
Fig. 8. T cell-induced Ccl5/CD44-mediated cell survival underlies Nf1 optic glioma growth.
a, b Immunohistochemistry revealed that anti-CD8 antibody treatment reduced the percentage of p-AKTSer473-expressing and p-CREBSer133-expressing cells in mouse Nf1 optic glioma specimens. Black arrows denote representative immunopositive cells. Scale bars, 20 µm. Bar graphs represent the means ± SEM of n = 4 independent biological samples. Two-tailed Students-t test. Exact P values are indicated within each panel; a P < 0.001; b P = 0.002. c, d Kaplan–Meier survival curves (Brain Lower Grade Glioma TCGA Provisional [2 left panels; c P = 1.29e−6, d P = 2.06e−3] and TCGA PanCancer Atlas [2 right panels; c P = 4.69e−13, d P = 9.86e−3] datasets) demonstrate that non-overlapping patients with LGG who harbor high CCL5 expression or CD44 expression have reduced survival time. e Schematic representation of the neuron–immune–cancer axis in NF1-LGG. Meningeal T cells infiltrate into the optic glioma in an integrin (VLA-4)-dependent manner, and are activated by MDK produced by Nf1-mutant retinal ganglion cells (neurons) through a RAS-dependent mechanism. This neuron-mediated T cell activation increases CD8+ T cell Ccl4 production through increased Lrp1/calcineurin signaling, and results in increased NFκB-dependent microglial Ccl5 expression, culminating in increased glioma growth through Akt/GSK3β/CREB pathway-mediated suppression of cancer (glioma) stem cell apoptosis and increased tumor growth.