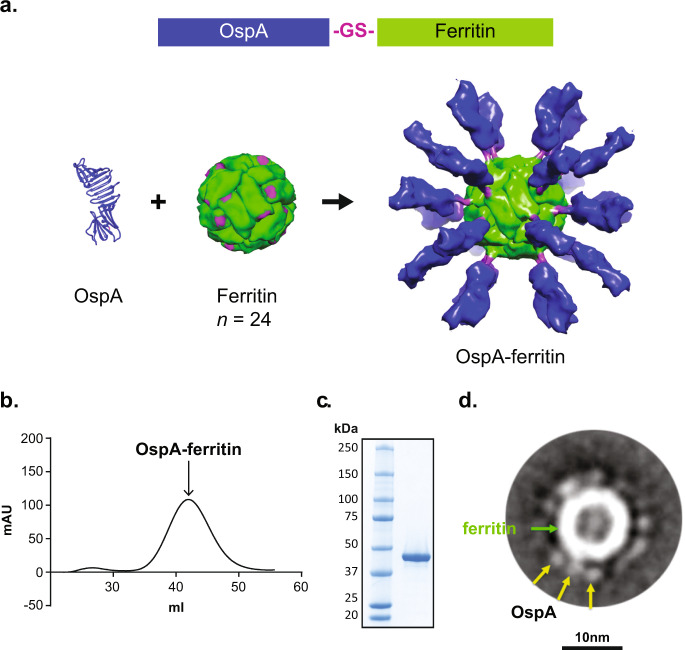
Fig. 1. Design, expression and purification of OspA-ferritin nanoparticles.
Schematic diagram of OspA fused to a modified H. pylori ferritin through a glycine–serine (GS) linker (A,top). Secondary structure of the transmembrane domain deletion of OspA, in which the carboxy-terminus of OspA where it is attached to ferritin is indicated (purple) (a, bottom left). The ferritin nanoparticle is composed of 24 monomers of H. pylori ferritin (a, bottom middle). The amino-terminal attachment site for OspA on ferritin is highlighted (purple). Structural model of the OspA-ferritin nanoparticle (a, bottom right). Ferritin (green), the GS linker (purple), and OspA (blue) is shown. A SEC profile of OspA-ferritin nanoparticle purification on a Superose 6 column (b). A SDS-PAGE gel of purified OspA-ferritin from E.coli (c). Annotated class averages of OspA-ferritin (318 particles) at 67,000x magnification (d). The ferritin cage appears as a strong circular density with a hollow center in the middle of the averages (green arrow). Each cage is surrounded by numerous, short, uniform spikes of OspA that appear circular or slightly oblong in shape (yellow).