Fig. 2. Multimerization of OsGA2ox3 enhances its activity in vitro and in planta.
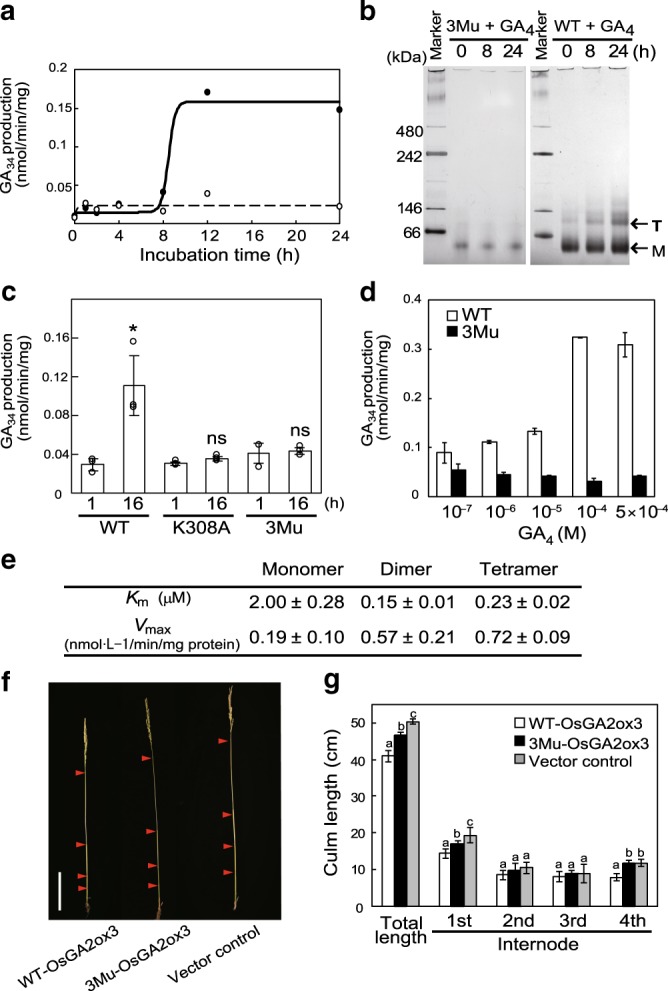
a Time course of OsGA2ox3 activation by GA4. The activity of WT- and 3Mu-OsGA2ox3 is shown by closed and open circles, respectively. b Assessment of OsGA2ox3 multimerization by blue-native PAGE. WT- and 3Mu-OsGA2ox3 are indicated with GA4 for 0, 8, and 24 h. Monomer (M) and tetramer (T) are indicated. c Enzyme analyses of OsGA2ox3, 3Mu, and K308A mutant (n = 3 biologically independent samples; central values and error bars represent means ± s.d.). The differences within the same category are indicated. *P < 0.05; NS not significant (P > 0.05); two-tailed paired t-tests. d Dose response of OsGA2ox3 and 3Mu activation by GA4 (n = 3 biologically independent samples; central values and error bars represent means ± s.d.). OsGA2ox3 (white bars) and 3Mu (black bars) were incubated for 12 h at various GA4 concentrations. e Km and Vmax of OsGA2ox3 with different multimerization forms (n = 3 biologically independent samples). f Representative internode elongation pattern shown in g. Red arrowheads indicate the position of nodes. Scale bar = 10 cm. g Complementation of the culm elongation in ga2ox3 knockout plants by overexpression of WT- and 3Mu-OsGA2ox3 cDNA (n = 6 biologically independent samples; central values and error bars represent means ± s.d.). Different letters denote significant differences (P < 0.05) based on the Tukey–Kramer test.
