Fig. 4. OsDAO forms an IAA-mediated dimer structure to trigger its enzyme activity.
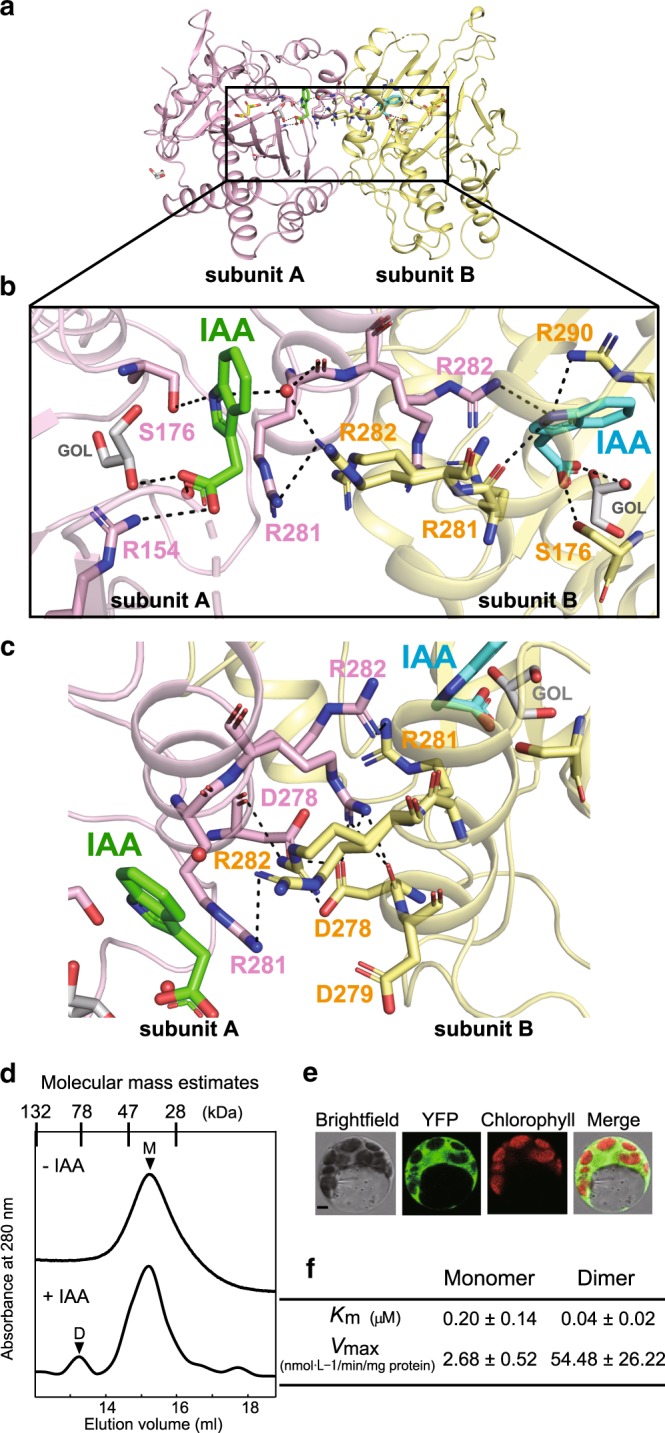
a Overall structure of the dimer form of OsDAO. b, c Detailed interactions at the dimer–dimer interface of the OsDAO bound to IAA (green and cyan stick forms), the residues surrounding the interface are represented by a stick model, shown as pink and yellow sticks. The hydrogen bonds mediated by water molecule (red spheres) are also indicated by dashed lines. d A typical elution profile of OsDAO sample under the presence of IAA condition in gel filtration. The proposed conformation of each eluted peak is indicated by M (monomer) and D (dimer). e BiFC analysis of dimeric interaction of DAO. Scale bar = 5 μm. f Kinetic analysis of monomer and dimer of OsDAO. Comparison of Km and Vmax calculated by the corresponding Lineweaver–Burk plots (n = 3 biologically independent samples).
