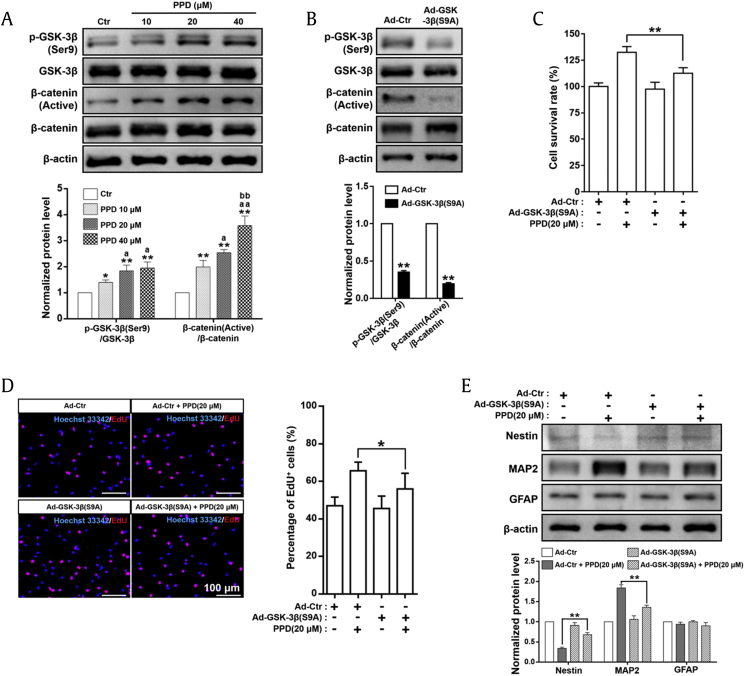
Fig. 5.
PPD-mediated NSC induction depends on the activation of the Wnt/GSK-3β/β-catenin signaling pathway. (A) Western blot analyses and relative optical density of diverse crucial markers in the Wnt/GSK-3β/β-catenin pathway in NSCs treated with PPD for 7 days. PPD significantly increased the p-GSK-3β(Ser9)/GSK-3β and β-catenin (active)/β-catenin ratios. *p < 0.05 and **p < 0.01, compared with the Ctr group; ap < 0.05 and aap < 0.01, compared with the 10 μM PPD group; bbp < 0.01, compared with the 20 μM PPD group. (B) NSCs with constitutively active GSK-3β were successfully established by GSK-3β(S9A) adenovirus transfection confirmed by Western blot analyses in NSCs 2 days after transfection. **p < 0.01, compared with the Ad-Ctr group. Control or GSK-3β(S9A) adenovirus were transfected into NSCs. Forty-eight hours after transfection, cells were treated with 20 μM PPD for further proliferation and differentiation assays. (C) Expression of the constitutively active GSK-3β in NSCs significantly decreased the cell viability induced by PPD as shown by CCK-8 assay. (D) Expression of the constitutively active GSK-3β in NSCs significantly restrained the proliferation induced by PPD as shown by EdU assay. (E) Expression of the constitutively active GSK-3β in NSCs significantly suppressed the PPD-mediated neural differentiation, determined by Western blot analyses of a variety of differentiation markers, including nestin, MAP2, and GFAP. *p < 0.05 and **p < 0.01, compared with the Ad-Ctr + PPD (20 μM) group. Data represent the means ± SD. PPD, 20(S)-protopanaxadiol; NSC, neural stem cell; GSK-3β, glycogen synthase kinase-3β; Ctr, control; Ad, adenovirus; MAP2, microtubule-associated protein 2; GFAP, glial fibrillary acidic protein; CCK-8, Cell Counting Kit-8; EdU, 5-ethynyl-2′-deoxyuridine; SD, standard deviation.