Fig. 4. Identification and characterization of Zap70 pTyr residues required for binding to the PKCθ C2 domain.
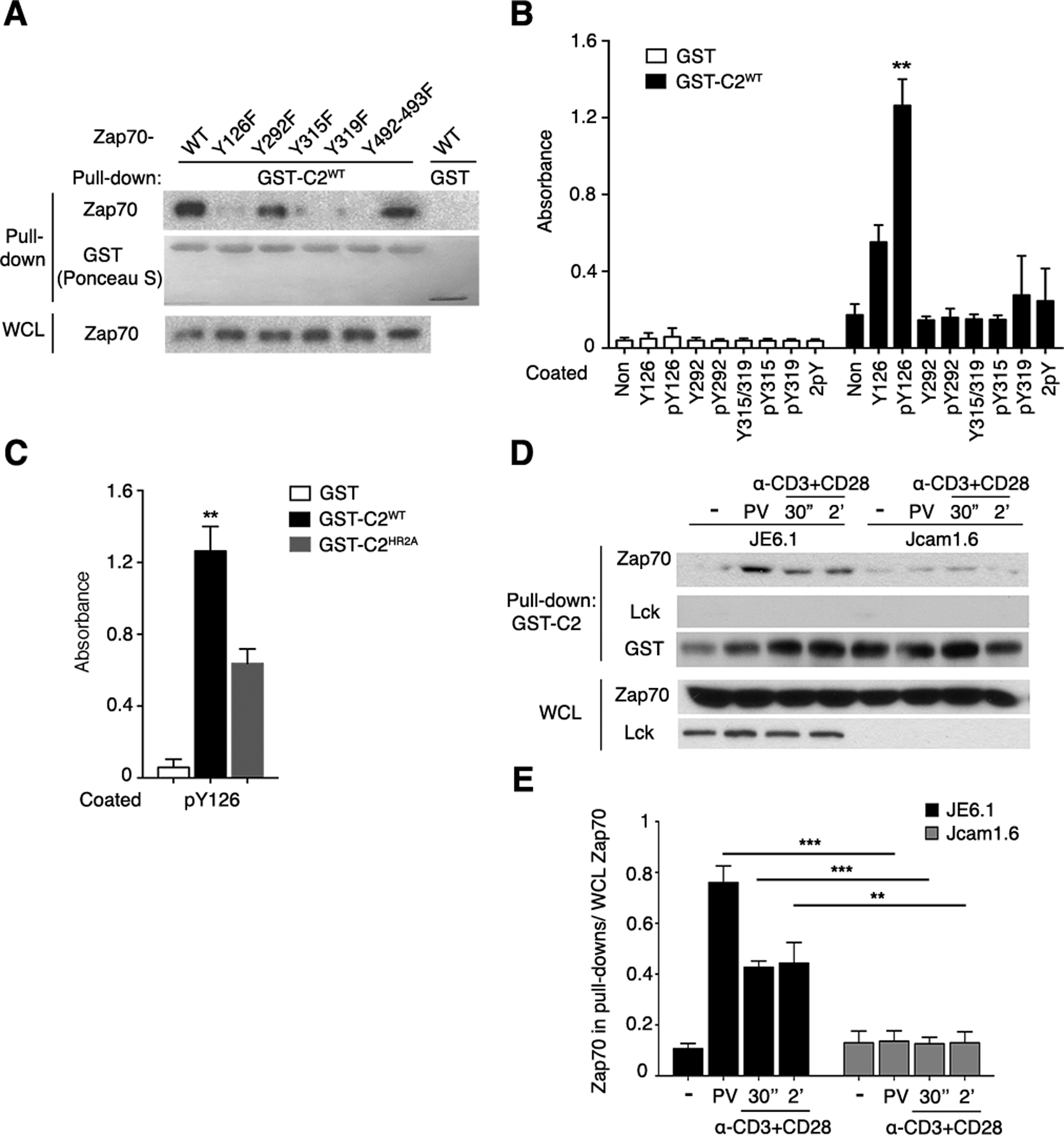
(A) Purified recombinant GST (control) or GST-C2WT was used to pull down proteins in lysates of PV-stimulated 293T cells expressing WT Zap70 or the indicated Zap70 mutants. Pulled-down material and whole-cell lysates (WCL) were separated by SDS-PAGE, stained with Ponceau S, and immunoblotted for Zap70. This experiment is representative of 3 independent experiments.
(B) Binding of recombinant GST and GST-C2WT to the indicated immobilized synthetic Zap70 peptides was assessed by an ELISA. Non, non-peptide-coated control wells.
(C) Binding of the indicated recombinant proteins to wells coated with the pTyr126 peptide determined by ELISA. Pooled data from 4 independent experiments are shown in (B) and (C), **P < 0.01.
(D and E) GST pull-down with lysates of WT (JE6.1) or Lck-deficient (Jcam1.6) Jurkat cells that were left unstimulated or stimulated with PV or CD3 and CD28 mAbs as indicated. C2WT-bound proteins or WCL were immunoblotted for the indicated proteins. Data are representative of 3 independent experiments. Pooled data from 3 independent experiments as (D) were quantitated by densitometry and shown in (E). **P < 0.01, ***P < 0.001.
