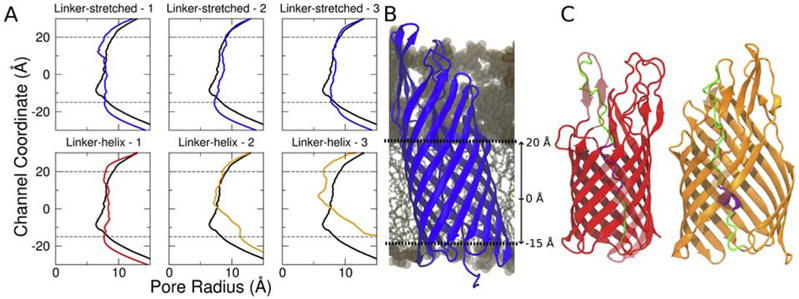
Figure 5:
The linker-helix EspP structure either unfolds the linker region or disrupts its β-barrel to secrete its passenger domain. (A) The EspP β-barrel pore sizes for both the linker-stretched and linker-helix EspP structure for three different replicas. The black line represents the initial pore size. The blue, red and orange lines represent the final pore size for each simulation. While both the linker-stretched replicas and one linker-helix do not demonstrate a significant pore size change over time, the other linker-helix replicas show pore expansion over time. (B) The channel coordinate position relative to the EspP β-barrel. (C) The mid-secretion structures of linker-native - 1 and linker-native - 2 (in red and in orange, respectively). In the initial 50 ns of simulation, linker-native - 1 unfolds the folded linker region and preserves its β-barrel structure (red). Linker-native - 2 and linker-native - 3 keep its linker region folded while disrupting the β-barrel structure (orange).