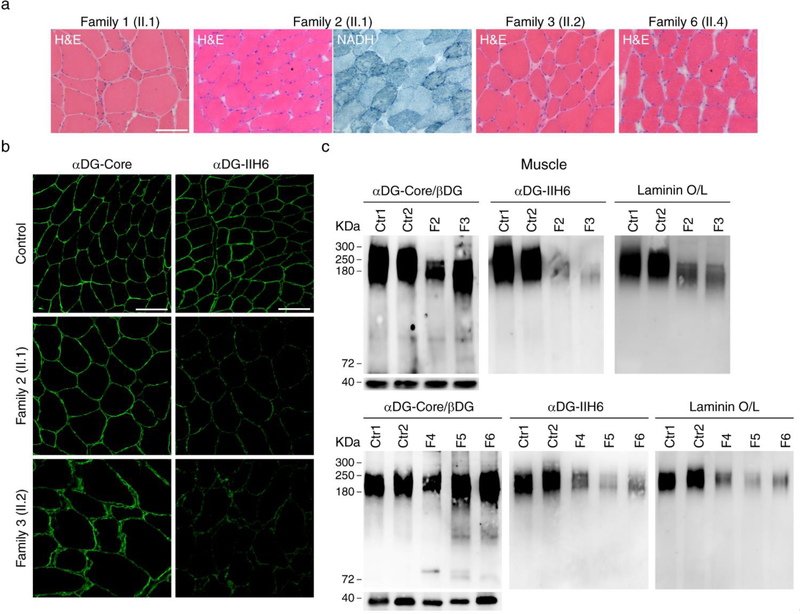
Figure 3. Decreased α-dystroglycan glycosylation and laminin binding in muscle.
a A biopsy from vastus lateralis muscle in patient II.1 from family 1 and II.2 from family 3 showed moderate dystrophic features with atrophic fibers and increased endomysial connective tissue; the muscle biopsies from biceps brachii in patient II.1 from family 2 and patient II.4 from Family 6 showed the usual myopathic features as internal nuclei and variable fiber size, together with lobulated fibers defined by small subsarcolemmal aggregates extending into the interior of the muscle fiber (asterisks); b immunostaning studies showed reduced signal using an antibody against glycosylated α-dystroglycan (IIH6) while the signal was the same as control samples using an antibody against the α-dystroglycan core, supporting α-dystroglycan hypoglycosylation; c Western blots corroborated the decreased α-dystroglycan glycosylation in all the muscle samples available, which showed a reduced level of glycosylated α-dystroglycan and low α-dystroglycan binding to laminin. Scale bar, 100 μm.