Figure 1.
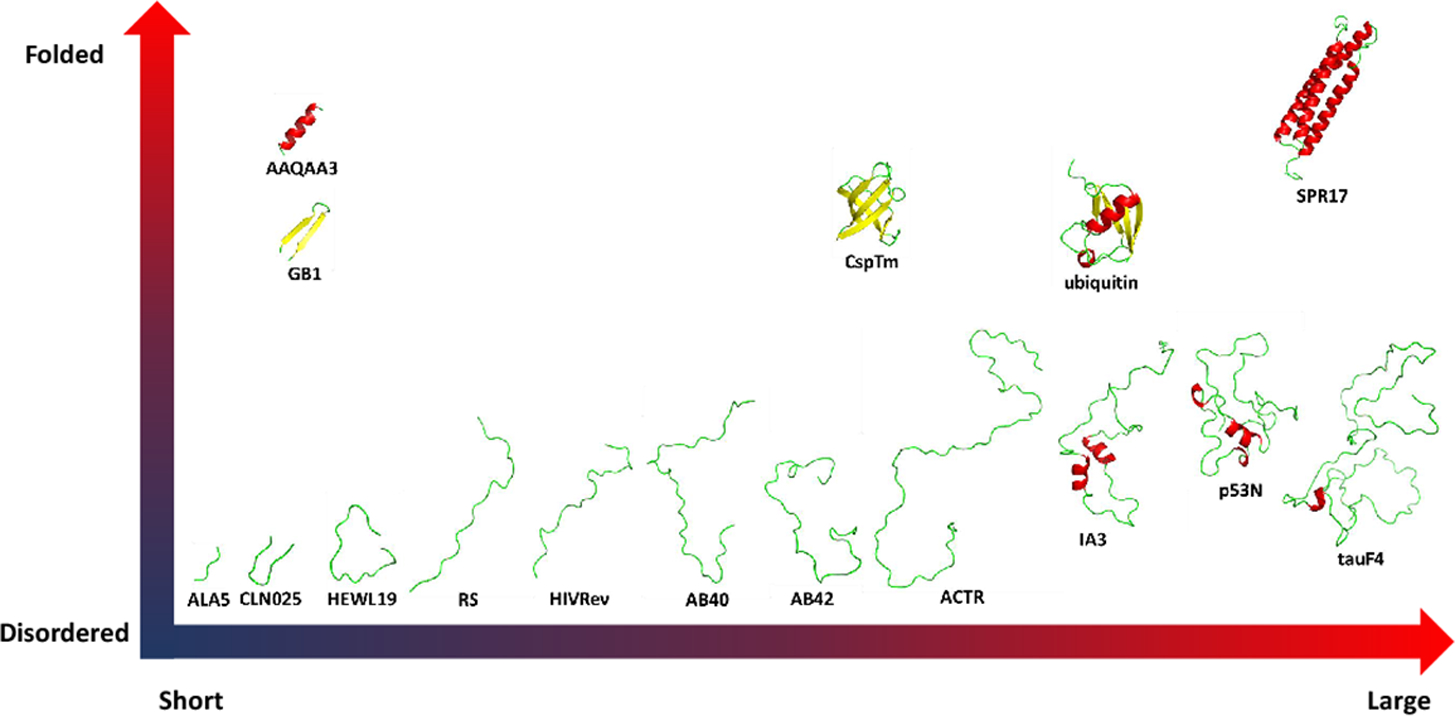
Test system from short peptides to proteins. Three representative folded proteins such as cold-shock protein from the hyperthermophilic bacterium Thermotoga maritima (CspTm, all-β),[23] ubiquitin of human (α/β)[24] and chicken brain alpha spectrin repeat 17 (SPR17, all-α).[25] 9 typical disordered protein including 19 length peptide of hen egg-white lysozyme (HEWL19),[26] phosphorylated SRSF1 (RS),[27] HIV-1 Rev ARM peptide (HIVRev),[28] 40 length amyloid-beta-peptides (AB40),[29] 42 length amyloid-beta-peptides (AB42),[29] activation domain of the nuclear hormone receptor coactivator (ACTR),[30] an aspartic proteinase inhibitor for Saccharomyces cerevisiae (IA3),[31] p53 N-terminal transactivation domain (p53N)[32] and tau protein fragment (TauF4).[33] 3 fast-folding proteins including 15-residue helix-forming peptide Ac-(AAQAA)3-NH2 (AAQAA3),[34] β-hairpin B1 domain of protein G (GB1)[35–36] and Chignolin, a 10 residue folded peptide designed by segment statistics (CLN025).[37]
