Summary
The World Health Organization has declared SARS-CoV-2 virus outbreak a worldwide pandemic. However, there is very limited understanding on the immune responses, especially adaptive immune responses to SARS-CoV-2 infection. Here, we collected blood from COVID-19 patients who have recently become virus-free, and therefore were discharged, and detected SARS-CoV-2-specific humoral and cellular immunity in eight newly discharged patients. Follow-up analysis on another cohort of six patients 2 weeks post discharge also revealed high titers of immunoglobulin G (IgG) antibodies. In all 14 patients tested, 13 displayed serum-neutralizing activities in a pseudotype entry assay. Notably, there was a strong correlation between neutralization antibody titers and the numbers of virus-specific T cells. Our work provides a basis for further analysis of protective immunity to SARS-CoV-2, and understanding the pathogenesis of COVID-19, especially in the severe cases. It also has implications in developing an effective vaccine to SARS-CoV-2 infection.
Keywords: SARS-CoV-2, COVID-19 patients, adaptive immunity, SARS-CoV-2-specific antibody, SARS-CoV-2-specific T cells
Highlights
-
•
SARS-CoV-2-specific antibodies are detected in COVID-19 convalescent subjects
-
•
Most COVID-19 convalescent individuals have detectable neutralizing antibodies
-
•
Cellular immune responses to SARS-CoV-2 are found in COVID-19 convalescent subjects
-
•
Neutralization antibody titers correlate with the numbers of virus-specific T cells.
In blood samples from COVID-19 convalescent subjects, Ni et al. have detected SARS-CoV-2-specific humoral and cellular immunity. Most subjects display serum neutralizing activities, which correlate with the numbers of virus-specific T cells.
Introduction
At the end of 2019, patients with coronavirus disease 2019 (COVID-19) were identified in Wuhan, China (Wang et al., 2020), infected by a novel coronavirus, now named as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The World Health Organization (WHO) first declared this outbreak a public health emergency of international concern (Phelan et al., 2020) and subsequently a worldwide pandemic (Di Pierro et al., 2020) .
The genome sequence of SARS-CoV-2 bears 96% (Zhou et al., 2020) and 79.5% identity to that of a bat coronavirus and SARS-CoV, respectively (Zhu et al., 2020). Like SARS-CoV and MERS-CoV, SARS-CoV-2 belongs to the beta genus Coronavirus in the Corornaviridae family (Lu et al., 2020). Clinically, several papers showed that most COVID-19 patients developed lymphopenia as well as pneumonia with higher plasma levels of pro-inflammatory cytokines in severe cases (Chan et al., 2020, Huang et al., 2020, Wu et al., 2020), suggesting that the host immune system is involved in the pathogenesis (Mahallawi et al., 2018, Nicholls et al., 2003). Patients infected by SARS-CoV or MERS-CoV were previously reported to have antibody responses (Ko et al., 2017, Shi et al., 2004, Wang et al., 2016, Woo et al., 2004) but exhibited defective expression of types I and II interferons (IFNs), indicative of poor protective immune responses (Cameron et al., 2008, Thiel and Weber, 2008, Vijay and Perlman, 2016). However, to date, there were few studies in characterizing the immune responses (Wolfel et al., 2020, Zhou et al., 2020), especially adaptive immune responses to SARS-CoV-2 infection. Zhou et al. showed that COVID-19 patients exhibited nucleocapsid protein (NP)-specific antibody response, and in one patient, immunoglobulin M (IgM) peaked at day 9 after disease onset and then switched to IgG by week 2 (Zhou et al., 2020). They also reported that sera from several patients could inhibit SARS-CoV-2 entry in target cells, indicating involvement of humoral immunity. Krammer and colleagues detected anti-S antibodies in three COVID-19 patients as early as 3 days post symptom onset (https://doi.org/10.1101/2020.03.17.20037713). A case report recently published showed the kinetics of T cell subpopulations (TFH, CD4 and CD8) and SARS-CoV-2-specific antibody responses in one COVID-19 patient (Thevarajan et al., 2020). One COVID-19 patient in Finland was shown to possess a low level of neutralizing antibody titer (Haveri et al., 2020). However, virus-specific T lymphocytes and their relationships with neutralizing antibody titers in COVID-19 patients remains uncharacterized.
In this study, we collected blood from COVID-19 patients who have recently become virus-free and therefore were discharged and analyzed their SARS-CoV-2-specific antibody and T cell responses.
Results
Detection of SARS-CoV-2-Specific Antibodies in COVID-19 Convalescent Subjects
To understand immune responses to COVID-19, we assessed 14 patients who recently recovered from the infection. Their clinical and pathological characteristics were shown in Table 1 . All the patients initially showed mild symptoms via computed tomography (CT) scan and were positive with SARS-CoV-2 nucleic acid testing. Of them, eight (patients 1–8) were newly discharged, whereas the remaining six were 2 weeks post discharge (follow-up patients, patients 9–14). Only three traveled to Wuhan city within the past 3 months. In line with the previous reports (Wang et al., 2016), two patients (5 and 10) showed lymphopenia (normal range is 1.1→3.2 × 10e9 cells per L). Sera from three healthy donors (Wang et al., 2016) were obtained before the SARS-CoV-2 outbreak (healthy donors 1–3). Three additional healthy donors (4–6) who had been in close contacts with the patients were recruited in this study. Human AB serum collected from healthy male AB donors in the United States (GemCell, CA) was used as a negative control.
Table 1.
Clinical and Pathological Characteristics of the COVID-19 Patients
| Pt# | Sex | Age | Travel in Wuhan | Fever | Fatigue | LymphocyteCount | Days in hospital | BT CT Scan | BT NA Test | Discharge CT Scan | Discharge NA Test |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 51 | yes | yes | yes | 1.1 × 109/L | 33 | patchy ground glass shadows on both lungs | P | improvement | N |
| 2 | F | 42 | no | no | no | 2.5 × 109/L | 27 | multiple patchy ground glass and high-density shadows in both lungs | P | improvement | N |
| 3 | M | 32 | no | yes | no | 1.7 × 109/L | 36 | exudative lesion of the right lower lung | P | improvement | N |
| 4 | M | 49 | no | yes | no | 1.5 × 109/L | 32 | patchy ground glass shadows on both lungs | P | significant improvement | N |
| 5 | F | 62 | no | yes | yes | 0.8 × 109/L | 37 | patchy ground glass shadows on both lungs | P | significant improvement | N |
| 6 | M | 32 | no | yes | yes | 2.1 × 109/L | 17 | multiple ground glass shadows in both lungs | P | significant improvement | N |
| 7 | M | 32 | no | yes | yes | 1.7 × 109/L | 34 | multiple ground glass lesions in the lower lobe of the right lung | P | significant improvement | N |
| 8 | F | 57 | yes | yes | yes | 1.3 × 109/L | 45 | multiple flaky ground glass shadows in the subpleural areas of both lungs, some accompanied by consolidation | P | significant improvement | N |
| 9 | F | 26 | no | yes | no | 2.9 × 109/L | 12 | right lung inflammation | P | normal | N |
| 10 | M | 68 | no | yes | no | 0.7 × 109/L | 14 | multiple patchy ground glass shadows are seen in the left lung, and the upper lobe of the left lung is obvious | P | significant improvement | N |
| 11 | F | 37 | no | no | yes | 1.9 × 109/L | 12 | double lung veins thickened | P | normal | N |
| 12 | F | 29 | no | yes | yes | 1.9 × 109/L | 13 | ground glass in the pleura of the lower lobe of both lungs | P | normal | N |
| 13 | F | 31 | yes | yes | no | 1.1 × 109/L | 19 | patchy ground glass shadows on both lungs | P | significant improvement | N |
| 14 | M | 35 | no | yes | yes | 2.3 × 109/L | 11 | multiple ground glass shadows in both lungs | P | normal | N |
Pt, patient; F, female; M, male; P, positive; N, negative; BT, before treatment; NA, nucleic acid.
In order to detect anti-viral immune responses, we first constructed recombinant pET28-N-6XHis by linking six copies of His tag to the C terminus of NP in the pET28-N vector (Biomed, cat. number BM2640). Escherichia coli transformed with pET28-N-6xHis was lysed and tested by Coomassie blue staining to confirm NP expression at 45.51 kDa. NP was further purified by Ni-NTA affinity chromatography and gel filtration. The purity of NP was approximately 90% (Figure S1A). The presence of NP was subsequently confirmed by anti-FLAG antibody (Figure S1B). The receptor-binding domain (RBD) of S protein (S-RBD) and main protease (Lan et al., 2020) were produced by a Baculovirus insect expression system and purified to a purity of 90% (Figure S1A).
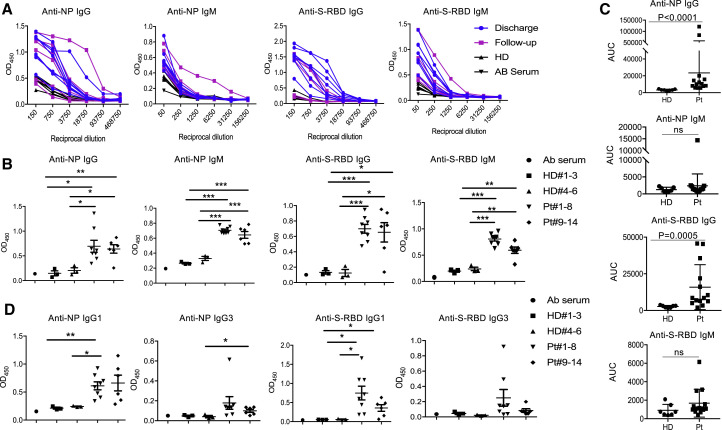
Using sera from patients and healthy donors, IgG and IgM against SARS-CoV-2 NP, main protease and S-RBD antigens were analyzed. There was no significant antibody response to main protease in sera from several patients (data not shown), suggesting that it may not serve as an antigen for humoral immunity. We thus focused on NP and S-RBD. The individual serum samples were then performed by serial dilutions to get optimal dilutions (Figure 1 A). Dilution of 1:50 was used for IgM and 1:450 for IgG. NP- and S-RBD-specific IgM and IgG antibodies were both detected in the sera of newly discharged patients, compared with healthy donor groups. Anti-SARS-CoV-2 IgG antibodies were also more obviously observed than IgM in the follow-up patients (9–14) when compared with healthy donors (Figure 1B). In addition, values from the serum dilution curves were calculated to determine the area under the curve (AUC) values. Compared to control sera, COVID-19 patient sera showed significantly higher AUC for NP- and S-RBD-specific IgG antibodies (Figure 1C). Taken together, these findings indicate that COVID-19 patients mounted IgG and IgM responses to SARS-CoV-2 proteins, especially NP and S-RBD, and also suggest that infected patients could maintain their IgG amounts, at least for 2 weeks after discharge.
Figure 1.
SARS-CoV-2 NP- and S-RBD-Specific Antibodies in COVID-19 Convalescent Individuals
(A) Titration of individual serum samples.
(B) Serological responses of 14 COVID-19 patients to recombinant NP (top) and S-RBD (bottom). Dilution of 1:50 was used for IgM and 1:450 for IgG.
(C) Data from the same experiments with (A) were presented as AUC.
(D) IgG isotypes of 14 COVID-19 patients to recombinant NP and S-RBD.
NP, nucleocapsid protein; S-RBD, receptor binding domain of spike protein; HD, healthy donor; Pt, patient; AUC, area under curve. The experiment was performed in duplicates. Date are presented as mean ± SEM. For HD 1–3, the sera were collected in 2018. For HD 4–6, the sera were from close contacts and collected in 2020. ∗p < 0.05, 0.05 < ∗∗p < 0.001, ∗∗∗p < 0.001.
In addition, IgG isotypes was further tested in 14 patients and 6 controls. As shown in Figure 1D, anti-NP and S-RBD IgG was mainly IgG1 isotype, and the newly discharged and follow-up patients showed similarly amounts of anti-NP IgG1. Of interest, one patient (5) showed higher amounts of anti-NP IgG3, whereas anti-S-RBD IgG3 was detected in two patients (4 and 5). However, we did not detect IgG2 to either NP or S-RBD proteins (data not shown).
Measurement of Neutralizing Antibody Titers from COVID-19 Convalescent Subjects
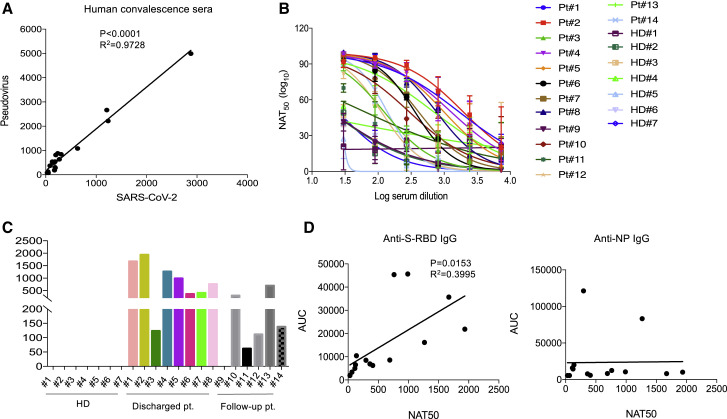
Since the RBD of the S protein has been shown to bind to human angiotensin converting enzyme 2 (ACE2) (Zhou et al., 2020), the existence of antibodies against it may suggest neutralization of SARS-CoV-2 infection. To assess this, we performed a pseudovirus particle-based neutralization assay, since there was a significantly positive correlation in the neutralizing antibody titers between pseudovirus and SARS-CoV-2 (Figure 2 A). As shown in Figures 2B and 2C, patients 1, 2, 4, 5, and 8, all within the newly discharged group, had high neutralizing antibody titers. These results demonstrate that most recently discharged patients had strong humoral immunity to SARS-CoV-2. Among the follow-up patients, all had neutralizing antibody titers, with the exception of patient 9 being negative. As expected, there was a significant correlation between neutralizing antibody titers and AUC of anti-S-RBD IgG, but not of anti-NP IgG (Figure 2D), suggesting that anti-S-RBD IgG might be predictive of serum neutralization capabilities in COVID-19 patients. These findings suggest that most patients post discharge have serum neutralizing SARS-CoV-2 infection.
Figure 2.
Measurement of Neutralizing Antibody Titers in COVID-19 Convalescent Individuals
(A) Correlation analysis of neutralizing antibody titers in COV1D-19 patients measured by pseudovirus and live SARS-CoV-2 (n = 20).
(B) Neutralizing curves of 14 COVID-19 patients measured by pseudovirus-based assay. The experiment with patients was performed in triplicates. The experiment with healthy donors was performed in duplicates.
(C) Measurement of neutralizing antibody titers of 14 COVID-19 patients by pseudovirus-based assay.
(D) Correlation between NAT50 and AUC of anti-S-RBD (left) and anti-NP (right) IgG (n = 14).
HD, healthy donor; Pt, patient; AUC, area under curve; NAT50, neutralizing antibody titers. Date are presented as mean ± SEM. ∗p < 0.05, 0.05 < ∗∗p < 0.001, ∗∗∗p < 0.001.
Cellular Immune Responses to SARS-CoV-2 in COVID-19 Convalescent Subjects
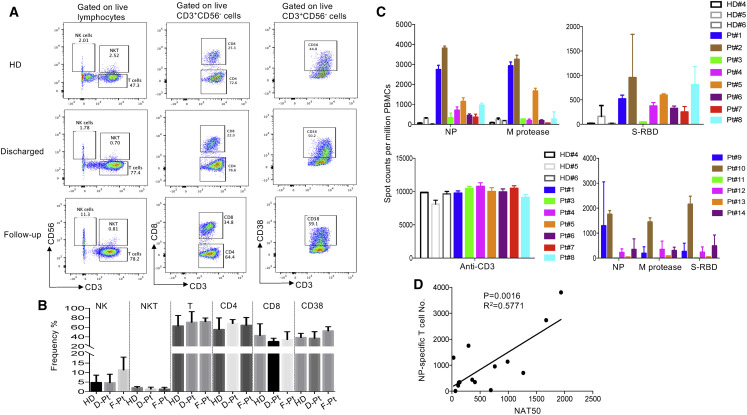
To explore cellular immune responses to SARS-CoV-2, we isolated peripheral blood monocytic cells (PBMCs) from the whole blood and phenotypically analyzed them by flow cytometry (Figure 3 A). We found that compared to newly discharged patients, there was a trend toward an increased frequency of NK cells in the follow-up patients (Figure 3B). However, there was no significant difference in terms of the percentages of T cells among those two groups and the healthy donors.
Figure 3.
T cell Responses to Recombinant SARS-CoV-2 Proteins in COVID-19 Convalescent Individuals
(A) Phenotypic analysis of PBMCs from representative COVID-19 patients.
(B) Summarized data on the frequencies of different immune cell subsets in COVID-19 patients. HD, healthy donors (n = 2); D-Pt, discharged patients (n = 3); F-Pt, follow-up patients (n = 5).
(C) IFN-γ ELISpot analysis of COVID-19 patients to recombinant proteins. The experiments were performed in duplicates.
(D) Correlation analysis of the NAT50 and the numbers of NP-specific T cells (n = 14).
M protease, main protease; NP, nucleocapsid protein; S-RBD, receptor binding domain of spike protein; NAT50, neutralizing antibody titers. Date are presented as mean ± SEM.
To assess virus-specific cellular immunity, we then treated PBMCs with recombinant NP, main protease, and S-RBD, followed by IFN-γ ELISpot analysis. The results were considered positive if there was at least a 2-fold increase in the numbers of IFN-γ-secreting T cells in the subject than in the healthy donors. As shown in Figure 3C, compared with healthy donors, the numbers of IFN-γ-secreting NP-specific T cells in patients 1, 2, 4, 5, and 8 were much higher than other patients, suggesting that they had developed SARS-CoV-2-specific T cell responses. Of note, patients 1, 2, 4, 5, and 8 developed both strong humoral and cellular immune responses. Main protease-specific T cells were detected in patients 1, 2, and 5, while patients 1, 2, 4, 5, 6, 7, and 8 showed S-RBD-specific T cells. Although the numbers of IFN-γ-secreting S-RBD specific T cells were much lower than those of NP-specific T cells, they could be detected in more patients than those for other viral proteins. In the follow-up patients, only patient 10, who showed lymphopenia before treatment, still had a high number of IFN-γ-secreting T cells in response to NP, main protease, and S-RBD (Figure 3C), which suggests that anti-viral T cells may not be maintained at high numbers in the PBMCs in the recovered patients. More interestingly, when combining all 14 patients in our analysis, there was a significant correlation between the neutralizing antibody titers and the numbers of NP-specific T cells (Figure 3D), indicating that the development of neutralizing antibodies may be correlated with the activation of anti-viral T cells. Thus, effective clearance of virus may need collaborative humoral and cellular immune responses.
Discussion
In this study, we characterized SARS-CoV-2-specific humoral and cellular immunity in recovered patients. Both were detected in newly discharged patients. In addition, the neutralizing antibody titers significantly correlated with the numbers of NP-specific T cells. These findings suggest both B and T cells participate in immune-mediated protection to viral infection. Our work has thus provided a basis for further analysis of protective immunity to SARS-CoV-2 and understanding the pathogenesis of COVID-19, especially in the severe cases. It has also implications in designing an effective vaccine to protect and treat SARS-CoV-2 infection.
In our study, production of S-RBD-specific antibodies were readily detected in recovered patients. Moreover, we observed virus-neutralization activities in these recovered patients. Not surprisingly, a significant correlation between neutralizing antibody titers and AUC of anti-S-RBD IgG, but not anti-NP IgG, was observed. Anti-S-RBD IgG might be useful in analyzing serum neutralization capabilities in COVID-19 patients. Our data are consistent with the work from other investigators (Zhou et al., 2020), in keeping with the role of humoral immunity in blockade of receptor binding during viral entry in host cells. Interestingly, S-RBD-specific T cell production of IFN-γ was also noted, suggesting that S-RBD also induced broader T cell immune responses. S-RBD, thus, is a promising target for SARS-CoV-2 vaccines.
Similar to a recent preprint (https://doi.org/10.1101/2020.03.30.20047365) published online after ours, the titers of neutralizing antibodies were variable in recovered patients, ranging from below detection (<30) to 1,936. Patient 9 did not exhibit significant serum virus-neutralizing activities. This patient, though with anti-NP and S-RBD IgM, did not have significant IgG or IgG1 production. Interestingly, this patient had detectable virus-specific T cell function. The basis for the neutralization deficiency in this patient and whether the patient can generate neutralizing antibodies thereafter needs further investigation. Nonetheless, in our study and the one mentioned above, most patients developed measurable neutralization antibodies after infection, suggesting that the viral infection does not curtail adaptive immunity. However, unlike the above-mentioned study, we did not find any correlation between neutralizing antibody titers and patients’ age, which could be due to our small sample size. Our results thus need further confirmation in a large cohort of COVID-19 patients. In addition, our analysis could not differentiate CD4+ and CD8+ T cell responses, due to the limitation in the amounts of PBMCs obtained and availability of instrumentation.
STAR★Methods
Key Resources Table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Anti-CD45 (clone H130) | BioLegend | Cat# 304028; RRID:AB_893338 |
| Anti-CD3 (clone OKT3) | BioLegend | Cat# 317334; RRID:AB_2561452 |
| Anti-CD8 (clone SK1) | BD Biosciences | Cat# 557834; RRID:AB_396892 |
| Anti-CD56 (clone HCD56) | BioLegend | Cat# 318304; RRID:AB_604100 |
| Anti-CD38 (clone HIT2) | BioLegend | Cat# 303508 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| Fixable viability dye eFluor660 | eBioscience | Cat# 65-0864 |
| FcR blocking reagent | Meltenyi Biotec, | Cat# 130-059-901 |
| Ficoll-Hypaque gradient | GE Healthcare Life | Cat# 17144002 |
| Goat anti-human IgM/HRP | Biosynthesis | Cat# bs-0345G-HRP; RRID:AB_10892735 |
| Goat anti-human IgG(biotin) | Sino Biological | Cat# SSA009 |
| HRP Mcab mouse anti-human IgG1 | BaiaoTong | Cat# C030248 |
| HRP Mcab mouse anti-human IgG2 | BaiaoTong | Cat# C030245 |
| HRP Mcab mouse anti-human IgG3 | BaiaoTong | Cat# C030246 |
| TMB substrate | Invitrogen | Cat# 00-4201-56 |
| Mouse anti-His monoclonal antibody | Proteintech | Cat# HRP-66005 |
| Recombinant His-tagged NP of SARS-CoV-2 | In-house | N/A |
| Recombinant His-tagged S-RBD of SARS-CoV-2 | Lan et al., 2020 | N/A |
| Recombinant main protease of SARS-CoV-2 | Lan et al., 2020 | N/A |
| Critical Commercial Assays | ||
| Fixation/Permeabilization Solution Kit | BD Biosciences | Cat# 554714 |
| Human IFN-γELISpotpro kit | MABTECH | Cat# 3420-2AST-2 |
| Biological Samples | ||
| Human AB serum | GemCell | Cat# 100-512 |
| Sera from HD#1-3 | In-house | N/A |
| Blood samples from HD#4-6 | ChuiYangLiu Hospital | N/A |
| Blood samples from COVID-19 patients | ChuiYangLiu Hospital | N/A |
| Experimental Models: Organisms/Strains | ||
| HuH-7 Cells | Nie et al., 2020 | N/A |
| Software and Algorithms | ||
| FlowJo software v10.7 | FlowJo LLC | https://www.flowjo.com/; RRID:SCR_008520 |
Resource Availability
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Chen Dong (chendong@tsinghua.edu.cn)
Materials Availability
The plasmid (pET28-N-6XHis) generated in this study will be made available on request from the Lead Contact without restriction.
Data and Code Availability
The study did not generate any unique dataset or code.
Experimental Model and Subject Details
COVID-19 patient blood samples
The blood samples of COVID-19 patients and healthy donors were obtained from Chui Yang Liu Hospital affiliated to Tsinghua University in Beijing. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (the institutional review board at Tsinghua University) and with the Helsinki Declaration of 1975, as revised in 2000. All studies were approved by the Medical Ethical Committee at Tsinghua University. Informed consent was obtained from all subjects for being included in the study. All patient data were anonymized before study inclusion. See Table 1 for full patient information, including age, sex, and health status.
Cell Lines
HuH-7 cells originally taken from a liver tumor in a Japanese male were cultured in DMEM supplemented with 10% FBS. Cells were grown at 37°C in a 5% CO2 setting.
Method Details
Expression and Purification of recombinant proteins
The recombinant His-tagged NP of SARS-CoV-2 was expressed in E. coli by a T7 expression system, with 1 mM IPTG induction at 37°C for 4 h. The recombinant His-tagged S-RBD (amino acids 319-541) was expressed by a Baculovirus system in insect cells (Lan et al., 2020). Purified proteins were identified by SDS-PAGE gels and stained with Coomassie blue. Western blot was performed to confirm their antigenicity by mouse anti-His monoclonal antibody (Proteintech, HRP-66005).
Isolation of PBMC
PBMCs were isolated from anti-coagulant blood using Ficoll-Hypaque gradients (GE Healthcare Life Sciences, Philadelphia, PA) as previously described (Xie et al., 2018) under the biosafety level 3 facility in AMMS. To isolate PBMCs, blood diluted with PBS, was gently layered over an equal volume of Ficoll in a Falcon tube and centrifuged for 30-40 min at 400-500 g without brake. Four layers formed, each containing different cell types. The second layer contained PBMCs. These cells could be gently removed using a Pasteur pipette and added to warm medium or PBS to wash off any remaining platelets. The pelleted cells were then counted and the percentage viability was estimated using Trypan blue staining. Cells were used immediately.
Anti-SARS-CoV-2 IgG/IgM ELISA
For IgM/IgG testing, 96-well ELISA plates were coated overnight with recombinant NP and S-RBD (100 ng/well). The sera from COVID-19 patients were incubated for 1 h at 37°C. An anti-Human IgG-biotin conjugated monoclonal antibody (Cat. SSA009, Sino Biological Inc., Wayne, PA) and streptavidin-HRP were used at a dilution of 1: 5000 and 1:250, respectively, and anti-human IgM-HRP conjugated monoclonal antibody (Cat. bs-0345G-HRP, Biosynthesis Biotechnology Inc. Beijing, China) was used. The OD value at 450 nm was calculated. The area under the curve (AUC) was calculated by Prism 7 (Graphpad).
Anti-SARS-CoV-2 IgG1/IgG2/IgG3 ELISA
For IgG1/IgG2/IgG3 test, 96 well ELISA plates were coated (80 ng/well) overnight with recombinant NP and S-RBD. Plates were washed and the sera from COVID-19 patients were incubated for 1 h at 37°C. After washing, an anti-Human IgG1-HRP conjugated monoclonal antibody (Cat. C030248, BaiaoTong Experiment Center, LY), an anti-human IgG2-HRP conjugated monoclonal antibody (Cat. C030245, BaiaoTong Experiment Center, LY) and an anti-human IgG3-HRP conjugated monoclonal antibody (Cat.C030246, BaiaoTong Experiment Center, LY), all validated by the company for their specificity, were used at a dilution of 1:4000 for 1 h at RT. After washing, TMB substrate solution was added. The OD value at 450 nm was calculated.
Neutralizing antibody assay
Pseudovirus expressing the SARS-CoV-2 S protein was produced as described previously (Deng et al., 1997). pNL43Luci and GP-pCAGGS were co-transfected into 293T cells. 48 h later, SARS-CoV-2 pseudovirus-containing supernatants were mixed with at least 6 serially diluted serum samples from the COVID-19 patients at 37°C for 1 h. Then the mixtures were transferred to 96-well plates containing monolayers of Huh-7 cells (Nie et al., 2020). 3 h later, the medium was replaced. After incubation for 48 h, the cells were washed, harvested in lysis buffer and analyzed for luciferase activity by the addition of luciferase substrate. Inhibition rate = [1-(the sample group- the cell control group) / (the virus control group- the cell control group)] x 100%. The neutralizing antibody titer (NAT50) were calculated by performing S-fit analysis via Graphpad Prism 7 software.
Interferon Gamma (IFN-γ) ELISpot
IFN-γ-secreting T cells were detected by Human IFN-γ ELISpotpro kits (MABTECH AB, Sweden) according to the manufacture protocol. Fresh PBMCs were plated in duplicate at 150k per well and then incubated 48 h with 1uM of recombinant proteins. Spots were then counted using an ELIspot Reader System (AT-Spot2100, atyx). The number of spots was converted into the number of spots per million cells and the mean of duplicate wells plotted.
FACS staining
PBMCs were washed with PBS plus 2% FBS (GIBCO, Grand Island, NY), and then Fc blocking reagent (Meltenyi Biotec, Inc., Auburn, CA) was added followed by a wash with PBS plus 2% FBS. Cells were then incubated for 30 min on ice with anti-CD45 (H130) (BioLegend), anti-CD3 (OKT3) (BioLegend), anti-CD8 (SK1) (BD), anti-CD56 (HCD56) (BioLegend), anti-CD38 (HIT2) (BioLegend) and live/dead fixable aqua dye (eF660, eBioscience), washed twice with PBS plus 2% FBS and then stored at 4°C until acquired by FACS Verse (BD Biosciences, San Jose, CA). Data were analyzed using FlowJo software (Version 10.0.8, Tree Star Inc., Ashland, Or).
Quantification and Statistical Analysis
Prism 7 software is used for statistical analysis. Student’s t test was performed for two-group analysis. Pearson’s correlation coefficients were calculated. P values less than 0.05 were considered to be statistically significant.
Acknowledgment
We thank Drs. Rong Mu and Zhinan Ying for sharing secondary detection antibodies. We also thank Weijin Huang and Jianhui Nie from the procuratorate for sharing the plasmids for pseudovirus package. This work was supported in part by grants from the National Key Research and Development Program of China (2016YFC0906200 to C.D., 2016YFC130390 to L.N., and 2020YFA0707800 to X.W.), Natural Science Foundation of China (31991173, 31821003, and 31991170 to C.D.), Beijing Municipal Science and Technology (Z181100001318007, Z181100006318015, and Z171100000417005 to C.D.), Zhejiang University Foundation (2020XGZX014 to C.D.), and award from Tsinghua University (to C.D.).
Author Contributions
L.N. and C.D designed the research and analyzed the data. F.Y., Y.D., P.L., H.G., and F.C. collected clinical specimens; M.C. did most of the experiments at a P3 laboratory. Y.F., H.Z., P.W., J.G., M.G., X.L., L.S., T. C., P.W., C.Z., R.Z., and X.W. performed some experiments or prepared key reagents; L.N, C.Q., and C.D. analyzed the results; and L.N. and C.D. wrote the manuscript.
Declaration of Interests
L.N., Y.F., W.P., and C.D. have filed a provisional patent on the methodology of detecting SARS-CoV-2-specific antibody responses.
Published: May 3, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.immuni.2020.04.023.
Contributor Information
Cheng-Feng Qin, Email: qincf@bmi.ac.cn.
Fang Chen, Email: anzhenchenfang@163.com.
Chen Dong, Email: chendong@tsinghua.edu.cn.
Supplemental Information
References
- Cameron M.J., Bermejo-Martin J.F., Danesh A., Muller M.P., Kelvin D.J. Human immunopathogenesis of severe acute respiratory syndrome (SARS) Virus Res. 2008;133:13–19. doi: 10.1016/j.virusres.2007.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J.F., Yuan S., Kok K.H., To K.K., Chu H., Yang J., Xing F., Liu J., Yip C.C., Poon R.W. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395:514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng H.K., Unutmaz D., KewalRamani V.N., Littman D.R. Expression cloning of new receptors used by simian and human immunodeficiency viruses. Nature. 1997;388:296–300. doi: 10.1038/40894. [DOI] [PubMed] [Google Scholar]
- Di Pierro F., Bertuccioli A., Cavecchia I. Possible therapeutic role of a highly standardized mixture of active compounds derived from cultured Lentinula edodes mycelia (AHCC) in patients infected with 2019 novel coronavirus. Minerva Gastroenterol. Dietol. 2020 doi: 10.23736/S1121-421X.20.02697-5. Published online March 12, 2020. [DOI] [PubMed] [Google Scholar]
- Haveri A., Smura T., Kuivanen S., Österlund P., Hepojoki J., Ikonen N., Pitkäpaasi M., Blomqvist S., Rönkkö E., Kantele A. Serological and molecular findings during SARS-CoV-2 infection: the first case study in Finland, January to February 2020. Euro Surveill. 2020;25:16–21. doi: 10.2807/1560-7917.ES.2020.25.11.2000266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko J.H., Müller M.A., Seok H., Park G.E., Lee J.Y., Cho S.Y., Ha Y.E., Baek J.Y., Kim S.H., Kang J.M. Serologic responses of 42 MERS-coronavirus-infected patients according to the disease severity. Diagn. Microbiol. Infect. Dis. 2017;89:106–111. doi: 10.1016/j.diagmicrobio.2017.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan J., Ge J., Yu J., Shan S., Zhou H., Fan S., Zhang Q., Shi X., Wang Q., Zhang L., Wang X. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 2020 doi: 10.1038/s41586-020-2180-5. Published online March 30, 2020. [DOI] [PubMed] [Google Scholar]
- Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., Wang W., Song H., Huang B., Zhu N. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahallawi W.H., Khabour O.F., Zhang Q., Makhdoum H.M., Suliman B.A. MERS-CoV infection in humans is associated with a pro-inflammatory Th1 and Th17 cytokine profile. Cytokine. 2018;104:8–13. doi: 10.1016/j.cyto.2018.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls J.M., Poon L.L., Lee K.C., Ng W.F., Lai S.T., Leung C.Y., Chu C.M., Hui P.K., Mak K.L., Lim W. Lung pathology of fatal severe acute respiratory syndrome. Lancet. 2003;361:1773–1778. doi: 10.1016/S0140-6736(03)13413-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie J., Li Q., Wu J., Zhao C., Hao H., Liu H., Zhang L., Nie L., Qin H., Wang M. Establishment and validation of a pseudovirus neutralization assay for SARS-CoV-2. Emerg. Microbes Infect. 2020;9:680–686. doi: 10.1080/22221751.2020.1743767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelan A.L., Katz R., Gostin L.O. Challenges for Global Health Governance. JAMA; China: 2020. The Novel Coronavirus Originating in Wuhan. [DOI] [PubMed] [Google Scholar]
- Shi Y., Wan Z., Li L., Li P., Li C., Ma Q., Cao C. Antibody responses against SARS-coronavirus and its nucleocaspid in SARS patients. J. Clin. Virol. 2004;31:66–68. doi: 10.1016/j.jcv.2004.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thevarajan I., Nguyen T.H.O., Koutsakos M., Druce J., Caly L., van de Sandt C.E., Jia X., Nicholson S., Catton M., Cowie B. Breadth of Concomitant Immune Responses Prior to Patient Recovery: A Case Report of Non-Severe COVID-19. Nat. Med. 2020;26:453–455. doi: 10.1038/s41591-020-0819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel V., Weber F. Interferon and cytokine responses to SARS-coronavirus infection. Cytokine Growth Factor Rev. 2008;19:121–132. doi: 10.1016/j.cytogfr.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijay R., Perlman S. Middle East respiratory syndrome and severe acute respiratory syndrome. Curr. Opin. Virol. 2016;16:70–76. doi: 10.1016/j.coviro.2016.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Wang H., Deng Y., Song T., Lan J., Wu G., Ke C., Tan W. Characterization of anti-MERS-CoV antibodies against various recombinant structural antigens of MERS-CoV in an imported case in China. Emerg. Microbes Infect. 2016;5:e113. doi: 10.1038/emi.2016.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Horby P.W., Hayden F.G., Gao G.F. A novel coronavirus outbreak of global health concern. Lancet. 2020;395:470–473. doi: 10.1016/S0140-6736(20)30185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfel R., Corman V.M., Guggemos W., Seilmaier M., Zange S., Müller M.A., Niemeyer D., Jones T.C., Vollmar P., Rothe C. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020 doi: 10.1038/s41586-020-2196-x. Published online April 1, 2020. [DOI] [PubMed] [Google Scholar]
- Woo P.C., Lau S.K., Wong B.H., Tsoi H.W., Fung A.M., Chan K.H., Tam V.K., Peiris J.S., Yuen K.Y. Detection of specific antibodies to severe acute respiratory syndrome (SARS) coronavirus nucleocapsid protein for serodiagnosis of SARS coronavirus pneumonia. J. Clin. Microbiol. 2004;42:2306–2309. doi: 10.1128/JCM.42.5.2306-2309.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F., Zhao S., Yu B., Chen Y.-M., Wang W., Song Z.-G., Hu Y., Tao Z.-W., Tian J.-H., Pei Y.-Y. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie S., Huang J., Qiao Q., Zang W., Hong S., Tan H., Dong C., Yang Z., Ni L. Expression of the inhibitory B7 family molecule VISTA in human colorectal carcinoma tumors. Cancer Immunol. Immunother. 2018;67:1685–1694. doi: 10.1007/s00262-018-2227-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., Si H.R., Zhu Y., Li B., Huang C.L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R., China Novel Coronavirus Investigating and Research Team A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The study did not generate any unique dataset or code.