Abstract
There are a number of cell therapies that are either in clinical trials or moving toward clinical trials, particularly for diseases of the retina. One of the challenges with cell therapies is tracking the status of cells over time. Genetic manipulation can facilitate this, but it can limit the clinical application of the cells. There are a host of fluorophores that have been developed to assess the status of cells, but these molecules tend to be cleared rapidly from cells. There are preclinical strategies that use degradable scaffolds, and we hypothesized that these scaffolds could be used to track the state of cells during preclinical studies. In this work, we explored whether fluorophores could be delivered from simple scaffolds fabricated under extremely harsh conditions, be active upon release, and report on the cells growing on the scaffolds over time. We encapsulated CellROX® Green Reagent, and pHrodo™ Red AM in poly(lactic-co-glycolic acid) (PLGA) scaffolds, showed that they could be delivered over weeks and were still active upon release and taken up by cells. These experiments provide the foundation for using scaffolds to deliver molecules to report on cells.
Key Terms: Polymer, retina, Age Related Macular Degeneration, AMD, cellular transplantation, tissue engineering, scaffold, polyester, drug delivery
Introduction
In the last decade, a number of cell therapies have moved to the clinic, particularly involving cell transplantation for diseases of retinal degeneration including retinitis pigmentosa (RP), diabetic retinopathy, and atrophic age-related macular degeneration (AMD) (1). Cell therapies offer the possibility of preserving and restoring vision in these conditions (2; 3). For example, human embryonic stem cell derived retinal pigment epithelial cells (RPE) have been transplanted in patients with AMD and led to improvements in vision (4). While this is tremendously promising, one of the challenges with preclinical research and clinical trials in this area is being able to track the behavior of the cells over time (2; 3). The eye provides a window, but there needs to be a way to determine the state of the cells. It is less than attractive to add extra reporter genes to the cells because it could impact the cell performance and safety in the clinic.
It is critical to the development of new therapies to be able to assess cell behavior over time. One option would be to use electronic monitoring of cells, and there has been some elegant work integrating electronics with scaffolds for electrochemical-based assessment (5; 6). Cells can be genetically engineered to express molecules to report on cell behavior (7; 8); however, genetic manipulation can limit subsequent clinical translation.
There are a range of fluorophores that can be added directly to cells to discern cell function and behavior (9) including molecules that respond to pH (10) and redox state (11). However, these molecules tend to be cleared rapidly from cells, especially retinal cells (12). There has been elegant work on developing molecules that remain in retinal cell membranes for long times, up to one month, but the vast majority of molecules are cleared within hours or days (13). For a reporter system to provide relevant data over time, ideally it would be reporting on the transplanted cells for at least one month if not longer since knowing the status of cells over time provides longitudinal data that is essential for understanding the connection between cell behavior, survival, and function in transplant settings.
Scaffolds are often used in conjunction with cellular transplantation in the eye to improve survival of the cells, to promote specific differentiation of cells, and to direct cellular architecture such as the alignment of photoreceptors and bipolar cells (14; 15). A number of these approaches are in preclinical or clinical phases of development (16; 17). Degradable polyesters such as poly(lactic-co-glycolic acid) (PLGA) are often used (18–20). While materials are not approved by regulatory agencies, approval is generally thought to be easier when using materials seen in previous applications in a tissue, and polyesters have a long history of use the eye (21). We hypothesized that the scaffolds could be used as a depot to deliver fluorophores to report on the state of cells, but there was concern about the organic solvents used to fabricate PLGA-based scaffolds and whether they would quench the activity of the fluorophores during the encapsulation process. There is limited data on how robust these reporter molecules are over time especially when subjected to adverse conditions like the presence of organic solvents used in many polymeric delivery systems.
We focused on using endothelial cells as a first cell for determining the viability of the reporter scaffold approach for two reasons. The first is that rat endothelial cells positively fluoresce with CellROX green and pHrodo red when they are proliferating without adding components which simplifies the approach significantly and removes a potential variable associated with adding extra components. The second is that endothelial cells are a critical cell type that is being considered for clinical trials with transplantation of new choroidal structures for the eye (17; 22) and endothelial cells are essential to the successful transplantation and behavior of retinal pigment epithelial (RPE) cells in clinical trials (4; 19). Further, endothelial cells and RPE cells often exhibit similar uptake behaviors motivating using one as a first step in characterizing the other’s performance (23).
The goal of the current study was to determine whether scaffolds could be loaded with reporter molecules that could be delivered over weeks and provide relevant data on cell behavior. As proof of principle experiments for embedding reporter molecules for long term assessment of cell function, we encapsulated CellROX® Green Reagent, and pHrodo™ Red AM in PLGA-based scaffolds to see if they could deliver active fluorophores over long times without significant changes to the processing to make the scaffolds which is often not particularly gentle to molecules.
Experimental Procedures
Materials
Two different commercially available reporter molecules were investigated as a first proof-of-concept: CellROX® Green (Invitrogen) is a cell permeable molecule based on fluorescein that weakly fluoresces green in a reduced state and increasingly fluoresces upon oxidation by reactive oxygen species (ROS). pHrodo™ Red AM (Invitrogen) is an intracellular pH indicator based on rhodamine that is weakly fluorescent at neutral pH and becomes increasingly fluorescent as the pH drops from neutral. PLGA (Resomer 502H, 503H, and 504H) was purchased from Evonik Industries. All reagents were ACS grade and were purchased from Fisher Scientific. All media and supplements were purchased from Invitrogen Technologies (Carlsbad, CA).
Fabrication of Films
PLGA 502H was dissolved in chloroform at 1% w/v ratio. To make the CellROX green films, 500 μL of CellROX green were added to 4 ml of the PLGA solution, and 300 μL were added to a standard glass slide, and the chloroform was allowed to evaporate in a chemical hood. To make the pHrodo red films, 4 μL of pHrodo red was added to 4 ml of the PLGA solution along with 40 μL of the powerload component based on the protocol provided by Invitrogen with the pHrodo red molecule. Following chloroform evaporation, films were gently removed from the glass and stored at −20 C and protected from light until use.
Coverslips
Coated coverslips were fabricated in a method similar to the films except that 12 mm coverslips were coated with 400 μL of the PLGA-dye solutions and then allowed to dry in the chemical hood before storing them at −20 C and protected from light until use.
Sponges
5% (w/v) PLGA in chloroform solutions were made. CellROX green was added with a 1.25% v/v ratio to the solution. pHrodo red was added in a .05% v/v ratio to the solution followed by 0.5% v/v powerload solution. 0.4 g of salt (250–300 μm size) was added to a container followed by 240 μL of the solution. The chloroform was allowed to evaporate in the chemical hood, and the salt was leached in water over 8 hours with 6 water changes. The scaffolds were dried and stored at −20 C until use.
Release Studies
For films, sections weighing approximately 120 mg were used. For sponges, sponges weighing approximately 40 mg were used. Polymers were placed in 1 ml of PBS at 37 C in triplicate. At each timepoint, the PBS was removed and stored at −20 C and replaced with fresh PBS to create an infinite sink release condition.
The amount of CellROX green and pHrodo red were measured using an M3 SpextraMax microplate reader (Molecular Devices, San Jose, CA). CellROX green was excited at 485 nm and the emission was measured at 520 nm. pHrodo red was excited at 560 nm and read at 585 nm.
Rat Endothelial Cell Culture
Rat endothelial cells were a generous gift from Prof. Joseph Madri. The cells have been isolated from rat fat pads and all animal procedures were approved by the Animal Care and Use Committee of Yale University (24). Endothelial cells were cultured in medium containing Dulbecco’s Modified Eagle’s Medium (DMEM), 10% fetal bovine serum (FBS), 10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), 1mM sodium pyruvate, 1mM L-glutamine, and 1% penicillin/streptomycin. Cells were grown on gelatin coated T75 flasks and passed at 1:4 when confluent.
Bioactivity of Reporter Molecules Following Release
Supernatants from the release study were sterile filtered using 0.25 μm filters and added to endothelial cells at a 1:20 dilution in media. Cells were imaged 3–4 hours after addition using an inverted fluorescence microscope (Zeiss Axio Observer Z1).
Growth of Cells on Films and Scaffolds Delivering Reporter Molecules
Films and sponges were sterilized by soaking in 70% ethanol overnight followed by 3 washes in PBS before cell seeding. Endothelial cells were seeded at 50,000 cells/ml on either scaffolds or films. Following 30 minutes for cell attachment to scaffolds, more media was added. Cultures were protected from light and kept in an incubator at 37 C and 5% CO2 except when imaged using a Zeiss Axio Observer Z1 microscope.
Thin Film-coated Coverslips for Long Term Cell Imaging
Coverslips were sterilized with UV-light for 30 minutes prior to seeding. Coverslips were placed in 12 well plates and endothelial cells were seeded at 50,000 cells/ml. Coverslips were imaged using a Zeiss Axio ObserverZ1 microscope. At the end of the experiment, the cells were fixed in 10% formalin, washed three times, and cover slipped with vectrashield containing DAPI for further imaging.
Results
The concept of this work was to determine the feasibility of encapsulating reporter molecules to report on the state of cells grown on scaffolds over time.
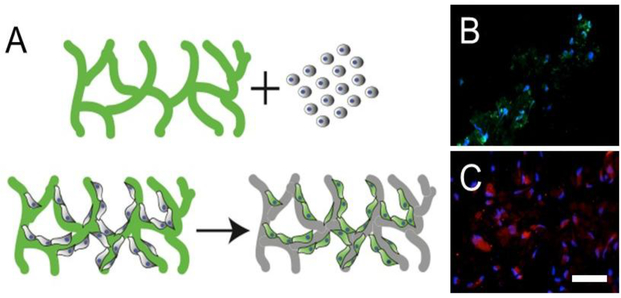
The molecules chosen for the work are commercial fluorophores from Molecular Probes (Invitrogen), CellROX green and pHrodo red. CellROX green is a cell permeable molecule that measures reactive oxygen species (ROS) in cells and fluoresces when it is oxidized. In the non-oxidized state, it does not fluoresce, but it emits as it is oxidized. pHrodo red fluoresces in response to a drop in pH and the fluorescence decreases as the pH increases. Endothelial cells are ideal for these experiments because they take up a wide range of reporter molecules including CellROX green and pHrodo red, and these cells are under oxidative stress and exhibit low pH while they are proliferating (Fig 1B and 1C). This makes them attractive as a platform for determining whether the use of reporter scaffolds is a viable approach.
Figure 1:
(A) Schematic of reporter scaffold concept delivering a molecule of interest (green) to cells over time (B) Endothelial cells fluorescing green when CellROX green is added to the live proliferating cells (C) Endothelial cells fluorescing red when pHrodo red is added to the live proliferating cells. Scale bar= 50 μm
Bioactivity of Molecules Released from Films
One of the first questions that needed to be addressed for the development of a reporter system was whether or not the activity of the molecules could be preserved upon encapsulation in and release from PLGA using organic solvents. PLGA films were fabricated using chloroform as the solvent because organics often quench the bioactivity of molecules (25).
Films were incubated at 37 C in PBS, and supernatant was collected at a range of timepoints. The concentration of reporter molecules released at the different timepoints was quantified, and the supernatant was added to endothelial cells to determine if the molecules were still able to fluoresce, even at the low concentrations released by the films at the longer timepoints.
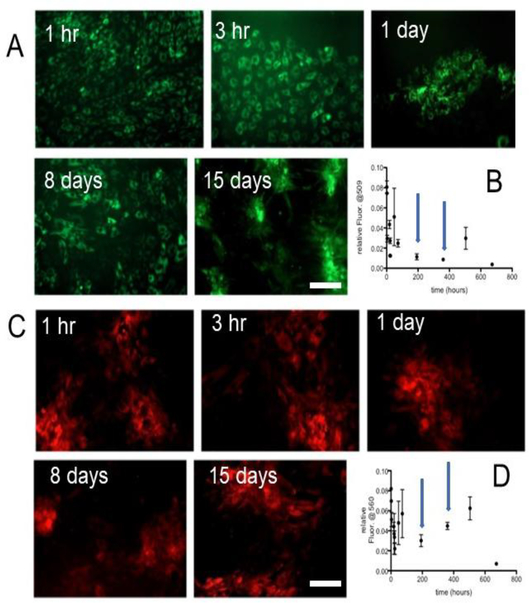
Figure 2 shows the endothelial cells incubated with CellROX green supernatant from multiple time points in the release study. Importantly, when the cells are under oxidative stress, they fluoresce just as they do when CellROX green is added directly to them. This data suggests that encapsulation and release is not affecting the biological activity of this molecule. The pHrodo red data follows the same trend. It is important to note that amount of reporter molecules in the supernatants is quite low, and there is no concentration step before adding the supernatant to the cells. In fact, the supernatant is added at a one to twenty dilution. Nonetheless, the molecules are reporting on the pH and oxidative stress of the cells as they should.
Figure 2:
(A) CellROX green released from the PLGA films from timepoints at 1 and 3 hours and 1,8, and 15 days demonstrate that the cells are under oxidative stress, even though the amounts being released by the films are small (B). The blue arrows mark the 8- and 15-day time points. (C) Likewise, the pHrodo red released from the PLGA films continues to report on the low intracellular pH, even though the amounts are small (D).
Growing Cells Directly on Coverslips Coated with PLGA-dye Combinations
Since it can be challenging to image cells on free-floating films, endothelial cells were cultured on coverslips coated with PLGA encapsulating the reporter molecules as a first step to determine whether cells could take up the dyes while growing on the materials.
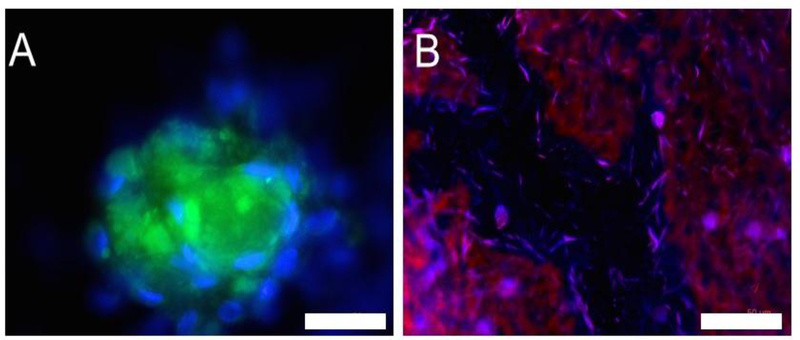
At 4 days post seeding, the cells took up the dyes and fluoresced. The cells were fixed and DAPI was applied to get a better sense of how many cells were fluorescing. A subset of the cells is positive for the reporter molecules which is consistent with previous experiments adding reporter molecules directly to the endothelial cells. This data demonstrates that the reporter molecules can be encapsulated in materials on which the cells grow and subsequently taken up by the cells. While polymer-coated coverslips allowed easy visualization, by 4 days, the polymers began to separate from the coverslips in a number of places, motivating moving to films and scaffolds for longer term cell culture experiments since they are more robust.
Reporting on Cells Grown Directly on Films
Endothelial cells were seeded on the films and observed over time. The cells attached and continued to grow throughout the experiments as seen with normal PLGA-based films. Figure 4 shows the cells on the films as well as cumulative release curves for the molecules from the films showing that lesser amounts are still being released at these longer timepoints.
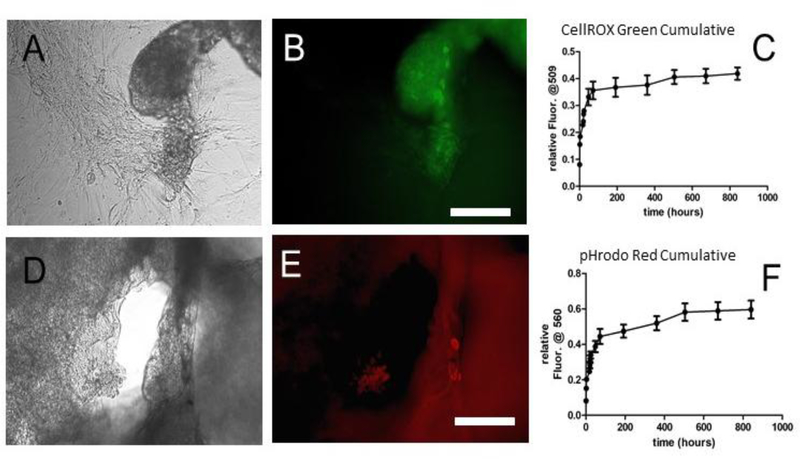
Figure 4:
Endothelial Cells were plated on films and imaged. At 34 days post seeding, some cells were still positive for CellROX Green (A, phase, B, fluorescence). The cumulative release curves of these films are shown in (C). Likewise, some endothelial cells grown on the films containing pHrodo Red were still positive at 34 days post seeding (D, phase, E, fluorescence). The cumulative release curve for pHrodo Red is in (F). Scale bar= 50 μm.
At 34 days post seeding, the cells could still be visualized. One of the challenges with working with free floating films, is that they tend to fold and wrinkle over time, and since these materials are degrading, they became very brittle by 34 days post seeding. Nonetheless, cells clearly took up the reporter molecules and fluoresced. As seen in the coverslip-coated study and consistent with the addition of these molecules to cells that are not proliferating rapidly, a subset of the cells fluoresces. However, the cells could be imaged (Figure 4), and cells positive for the reporter molecules were seen.
Three-dimensional Reporter Scaffolds
With the data suggesting that reporter molecules were active upon release and could be delivered to cells growing on materials, we synthesized scaffolds to look at the viability of the approach on 3D systems. The scaffolds were based on using PLGA and chloroform because the conditions tend to reduce bioactivity of a number of molecules including fluorophores.
Three different PLGAs were used with a range of molecular weights. PLGA 502H has a molecular weight of approximately 10kDa, 503H is closer to 22kDa, and 504H is approximately 35kDa. Endothelial cells were seeded on 502H sponges and imaged the cells over time.
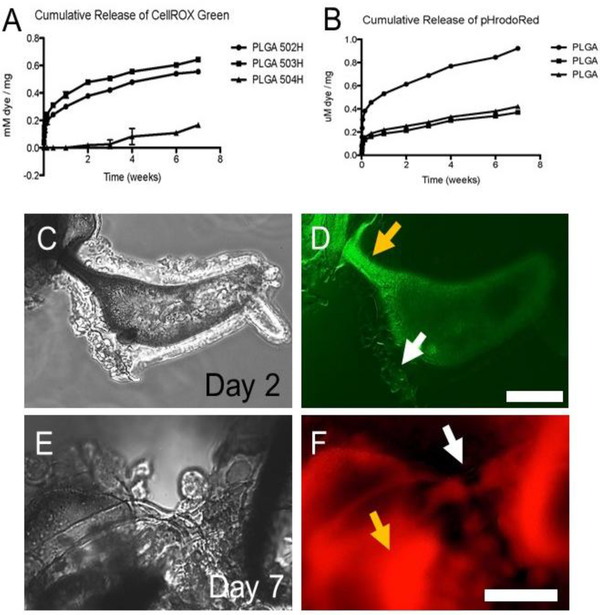
Figure 5 shows the release curves for CellROX green (A) and pHrodo red (B). Varying the polymer impacted the release for both reporter molecules with the general release profile being similar as we have seen for a number of other molecules (26; 27). These PLGAs have carboxylic acid groups on one chain end, and lower molecular weight polymers have an enrichment of carboxylic acid groups per volume of polymer which may augment the loading of a range of molecules, including the two reporter molecules studied here.
Figure 5:
3D salt leached scaffolds with reporter molecules. (A) Cumulative release of CellROX green as a function of PLGA. (B) Cumulative release of pHrodo red from salt leached scaffolds over time as a function of PLGA. All of the scaffolds released reporter molecules for 7 weeks. (C-F) Endothelial cells were grown on the 502H scaffolds. At 2 days post seeding, endothelial cells could be seen in phase (C) and some expressed CellROX green (D). Scale bar=50 μm. However, relatively few cells appeared to be positive for oxidative stress, and by 7 days, we did not see signs of cells positive for oxidative stress. At 7 days post seeding, we still found cells in phase (E) that were also positive for pHrodo red (F), but we did not find cells clearly positive for it after the 7-day timepoint. Scale bar=20 μm. Yellow arrows mark autofluorescence from polymer versus white arrows marking fluorescing cells.
The release curves suggest that simple scaffolds can deliver reporter molecules for several weeks. However, growing cells on the scaffolds and imaging them brings certain challenges. We used a standard epifluorescence microscope, and one of the biggest challenges with PLGA scaffolds is that the polymers autofluoresce particularly in thicker sections of the material. It can be challenging to distinguish cells from the scaffolds in places. However, near the large, open pores of these materials, we were able to find and image cells with some positive fluorescence for CellROX green at the early timepoints, of up to 2 days post seeding. pHrodo red, positive fluorescence up to 7 days post seeding. We did not see cells positive for either reporter molecule after these time points which follows the trends we saw for films, namely that as cells appeared to be confluent, the fluorescence decreased. One could certainly use a confocal or laser scanning system to manage the autofluorescent component, but it is something that should be considered in future scaffold designs with reporter molecules to optimize the system for tracking the status of cells.
Discussion
According to the World Glaucoma Association, people fear loss of vision more than death, in part because loss of vision dramatically alters one’s life and one’s independence and ability to interact on many levels (28). Across the Globe, approximately 8% of the population is likely to have AMD as they age with a substantial fraction progressing towards vision loss over time (29). Right now, there are a number of technologies using stem cells in the clinic for retinal degenerative conditions like AMD including RPE-scaffold transplants (4; 19) and stem cell/progenitor therapies more broadly (1; 4; 30). In many cases, a challenge lies with being able to assess the state of cells in the eye over time. Non-invasive, functional testing like electroretinograms is the norm, but it is hard to correlate these findings with information regarding the state of the cells in living organisms. The eye is a fantastic window for observing fluorescent signals, but genetically encoding for fluorescent proteins alters the cells and may limit translation. As new therapies and improvements on existing clinical therapies are developed, especially those with a combination of cells and scaffolds, we wanted to probe whether reporter molecules could be combined with scaffolds and would be taken up by the cells with the hope that this will facilitate tracking cell behavior over time to make these therapies as effective and robust as possible.
Reporter molecules can be incorporated into scaffolds and can report on the status of cells over time. The commercially available reporter molecules are robust and bioactive for long times, out to at least 34 days in some cases. Nonetheless, we do see relatively few cells that are positive over time on the scaffolds. Looking at how these molecules work, it is not surprising. CellROX green is a cell permeable molecule that measures reactive oxygen species (ROS) in cells by fluorescing when it is oxidized. ROS levels are elevated in endothelial cells during proliferation and migration (31; 32). Therefore, it makes sense that the cells fluoresced green when proliferating and migrating, but the fluorescence dropped once the cells were confluent. pHrodo red fluoresces in response to a drop in pH which occurs for a number of reasons including upon internalization in cell endosomes. When endothelial cells proliferate, they are far more likely to internalize molecules (33). While we could have pursued adding external ROS agents or promoting endocytosis of pHrodo red, we decided to focus on the simplest approach from the cell point of view as a proof of principle. Based on the supernatant studies, the molecules are active over time which is encouraging.
The principles developed in the current study involving the delivery of reporter molecules over time can also be applied to a range of cellular therapies by either incorporating them in appropriate scaffolds, or in cases where a scaffold is not desired, by co-injecting nanoparticles to deliver these molecules. This work provides a framework for engineering a completely new field of reporter scaffolds for assaying cells in vitro and in vivo.
To move this to a practical application, the appropriate scaffold would need to be used and the issue with autofluorescence would need to be addressed either by material choice, wavelength for the reporter molecule, architecture of the scaffold, or imaging modality. Nonetheless, it is striking that even with these far from ideal materials, scaffolds, and cell conditions without external promoters of ROS or internalization, we still were able to see these molecules report on the cells. The concept is sound, but the execution for a clinically relevant system would require consideration of the scaffold, molecule, and imaging system. For an application like an implant in the eye, the thin, organized scaffolds being used would likely lend themselves well to incorporation of reporter molecules (16; 17; 34).
In recent years many reporter molecules have been developed to inform on an array of cellular behaviors (10; 11; 35). As researchers develop tissue engineering constructs for a variety of applications, this reporter scaffold approach could provide valuable information on cellular behaviors to guide the design process and assess outcomes.
Figure 3:
Endothelial cells cultured on PLGA-coated coverslips delivering either CellROX green (A) or pHrodo red (B) at 4 days post seeding. Growing cells directly on the film-coated coverslips facilitates easy visualization of the cells. Scale bars: 50 μm.
Acknowledgements
This work was supported by a grant from the Steven J. Ryan Initiative for Macular Research and NIH grant 1R56NS100732-01. Dr. Lavik is an inventor on intellectual property that includes the potential for incorporation of reporter molecules.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.Tang Z, Zhang Y, Wang Y, Zhang D, Shen B, et al. 2017. Progress of stem/progenitor cell-based therapy for retinal degeneration. J Transl Med 15:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen Z, Zhang YA. 2015. Cell therapy for macular degeneration-first phase I/II pluripotent stem cell-based clinical trial shows promise. Science China. Life sciences 58:119–20 [DOI] [PubMed] [Google Scholar]
- 3.Zarbin M 2016. Cell-Based Therapy for Degenerative Retinal Disease. Trends in molecular medicine 22:115–34 [DOI] [PubMed] [Google Scholar]
- 4.da Cruz L, Fynes K, Georgiadis O, Kerby J, Luo YH, et al. 2018. Phase 1 clinical study of an embryonic stem cell-derived retinal pigment epithelium patch in age-related macular degeneration. Nat Biotechnol 36:328–37 [DOI] [PubMed] [Google Scholar]
- 5.Hu XB, Liu YL, Wang WJ, Zhang HW, Qin Y, et al. 2018. Biomimetic Graphene-Based 3D Scaffold for Long-Term Cell Culture and Real-Time Electrochemical Monitoring. Anal Chem 90:1136–41 [DOI] [PubMed] [Google Scholar]
- 6.Dai X, Hong G, Gao T, Lieber CM. 2018. Mesh Nanoelectronics: Seamless Integration of Electronics with Tissues. Accounts of chemical research 51:309–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoekstra ME, Dijkgraaf FE, Schumacher TN, Rohr JC. 2015. Assessing T lymphocyte function and differentiation by genetically encoded reporter systems. Trends in immunology 36:392–400 [DOI] [PubMed] [Google Scholar]
- 8.Liu Y, Deng W. 2016. Reverse engineering human neurodegenerative disease using pluripotent stem cell technology. Brain Res 1638:30–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ueda T, Tamura T, Hamachi I. 2018. In Situ Construction of Protein-Based Semisynthetic Biosensors. ACS sensors 3:527–39 [DOI] [PubMed] [Google Scholar]
- 10.Aziz M, Yang WL, Wang P. 2013. Measurement of phagocytic engulfment of apoptotic cells by macrophages using pHrodo succinimidyl ester. Current protocols in immunology Chapter 14:Unit 14.31. [DOI] [PubMed] [Google Scholar]
- 11.Yokoyama C, Sueyoshi Y, Ema M, Mori Y, Takaishi K, Hisatomi H. 2017. Induction of oxidative stress by anticancer drugs in the presence and absence of cells. Oncology letters 14:6066–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khoh-Reiter S, Sokolowski SA, Jessen B, Evans M, Dalvie D, Lu S. 2015. Contribution of membrane trafficking perturbation to retinal toxicity. Toxicological sciences: an official journal of the Society of Toxicology 145:383–95 [DOI] [PubMed] [Google Scholar]
- 13.Tochitsky I, Trautman J, Gallerani N, Malis JG, Kramer RH. 2017. Restoring visual function to the blind retina with a potent, safe and long-lasting photoswitch. Scientific reports 7:45487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Treharne AJ, Grossel MC, Lotery AJ, Thomson HA. 2011. The chemistry of retinal transplantation: the influence of polymer scaffold properties on retinal cell adhesion and control. Br J Ophthalmol 95:768–73 [DOI] [PubMed] [Google Scholar]
- 15.Kador KE, Goldberg JL. 2012. Scaffolds and stem cells: delivery of cell transplants for retinal degenerations. Expert review of ophthalmology 7:459–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tan YSE, Shi PJ, Choo CJ, Laude A, Yeong WY. 2018. Tissue engineering of retina and Bruch’s membrane: a review of cells, materials and processes. Br J Ophthalmol 102:1182–7 [DOI] [PubMed] [Google Scholar]
- 17.Sharma R, Khristov V, Rising A, Jha BS, Dejene R, et al. 2019. Clinical-grade stem cell-derived retinal pigment epithelium patch rescues retinal degeneration in rodents and pigs. Sci Transl Med 11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mazumder MA, Fitzpatrick SD, Muirhead B, Sheardown H. 2012. Cell-adhesive thermogelling PNIPAAm/hyaluronic acid cell delivery hydrogels for potential application as minimally invasive retinal therapeutics. J Biomed Mater Res A 100:1877–87 [DOI] [PubMed] [Google Scholar]
- 19.Carr AJ, Smart MJ, Ramsden CM, Powner MB, da Cruz L, Coffey PJ. 2013. Development of human embryonic stem cell therapies for age-related macular degeneration. Trends Neurosci 36:385–95 [DOI] [PubMed] [Google Scholar]
- 20.Kador KE, Montero RB, Venugopalan P, Hertz J, Zindell AN, et al. 2013. Tissue engineering the retinal ganglion cell nerve fiber layer. Biomaterials 34:4242–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bourges JL, Gautier SE, Delie F, Bejjani RA, Jeanny JC, et al. 2003. Ocular drug delivery targeting the retina and retinal pigment epithelium using polylactide nanoparticles. Invest Ophthalmol Vis Sci 44:3562–9 [DOI] [PubMed] [Google Scholar]
- 22.Bharti K 2018. Patching the retina with stem cells. Nat Biotechnol 36:311–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hafeli UO, Riffle JS, Harris-Shekhawat L, Carmichael-Baranauskas A, Mark F, et al. 2009. Cell uptake and in vitro toxicity of magnetic nanoparticles suitable for drug delivery. Mol Pharm 6:1417–28 [DOI] [PubMed] [Google Scholar]
- 24.Madri J, Williams S. 1983. Capillary Endothelial Cell Cultures: Phenotypic Modulation by Matrix Components. The Journal of Cell Biology 97:153–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sturesson C, Carlfors J. 2000. Incorporation of protein in PLG-microspheres with retention of bioactivity. J Control Release 67:171–8 [DOI] [PubMed] [Google Scholar]
- 26.Groynom R, Shoffstall E, Wu LS, Kramer RH, Lavik EB. 2015. Controlled release of photoswitch drugs by degradable polymer microspheres. J Drug Target 23:710–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lavik E, Kuehn MH, Shoffstall AJ, Atkins K, Dumitrescu AV, Kwon YH. 2016. Sustained Delivery of Timolol Maleate for Over 90 Days by Subconjunctival Injection. J Ocul Pharmacol Ther 32:642–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bramley T, Peeples P, Walt JG, Juhasz M, Hansen JE. 2008. Impact of vision loss on costs and outcomes in medicare beneficiaries with glaucoma. Arch Ophthalmol 126:849–56 [DOI] [PubMed] [Google Scholar]
- 29.Wong WL, Su X, Li X, Cheung CM, Klein R, et al. 2014. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. The Lancet. Global health 2:e106–16 [DOI] [PubMed] [Google Scholar]
- 30.Liu Y, Chen SJ, Li SY, Qu LH, Meng XH, et al. 2017. Long-term safety of human retinal progenitor cell transplantation in retinitis pigmentosa patients. Stem cell research & therapy 8:209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim YM, Kim SJ, Tatsunami R, Yamamura H, Fukai T, Ushio-Fukai M. 2017. ROS-induced ROS release orchestrated by Nox4, Nox2, and mitochondria in VEGF signaling and angiogenesis. Am J Physiol Cell Physiol 312:C749–c64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Y, Zang QS, Liu Z, Wu Q, Maass D, et al. 2011. Regulation of VEGF-induced endothelial cell migration by mitochondrial reactive oxygen species. Am J Physiol Cell Physiol 301:C695–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weber D, Torger B, Richter K, Nessling M, Momburg F, et al. 2018. Interaction of Poly(l-lysine)/Polysaccharide Complex Nanoparticles with Human Vascular Endothelial Cells. Nanomaterials (Basel, Switzerland) 8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Worthington KS, Wiley LA, Kaalberg EE, Collins MM, Mullins RF, et al. 2017. Two-photon polymerization for production of human iPSC-derived retinal cell grafts. Acta Biomater 55:385–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kapellos TS, Taylor L, Lee H, Cowley SA, James WS, et al. 2016. A novel real time imaging platform to quantify macrophage phagocytosis. Biochem Pharmacol 116:107–19 [DOI] [PMC free article] [PubMed] [Google Scholar]