Abstract
Rationale
Methamphetamine (METH) enhances exocytotic dopamine (DA) signals and induces DA transporter (DAT) mediated efflux in brain striatal regions such as the nucleus accumbens (NAc). Blocking sigma receptors prevents METH-induced DA increases. Sigma receptor activation induces Ca2+ release from intracellular stores, which may be responsible for METH-induced DA increases.
Objectives
The role of intracellular and extracellular Ca2+ on METH-induced DA increases and associated behavior was tested.
Methods
METH-induced Ca2+ release was measured in hNPC derived DA cells using ratiometric Ca2+ imaging. In mouse brain slices, fast-scan cyclic voltammetry was used to measure METH effects on two measures of dopamine: electrically stimulated and DAT mediated efflux. Intracellular and extracellular Ca2+ was removed through pharmacological blockade of Ca2+ permeable channels (Cd2+ and IP3 sensitive channels), intracellular Ca2+ chelation (BAPTA-AM) or non-inclusion (zero Ca2+). Last, METH effects on dopamine-mediated locomotor behavior was tested in rats. Rats received intra-NAc injections of ACSF or 2-aminoethoxydiphenyl borate (2-APB; IP3 receptor blocker) and intraperitoneal METH (5 mg/kg) to test the role of intracellular Ca2+ release in DA-mediated behaviors.
Results
Reducing Ca2+ extracellular levels and Ca2+ release from intracellular stores prevented intracellular Ca2+ release. Intracellular Ca2+ chelation and blocking intracellular Ca2+ release reduced METH effects on voltammetric measures of dopamine. Blocking intracellular Ca2+ release via 2-APB resulted in increased METH-induced circling behavior.
Conclusions
METH induces NAc DA release through intracellular Ca2+ activity. Blocking intracellular Ca2+ release prevents METH effects on DA signals and related behavior.
Keywords: Methamphetamine, Dopamine, Voltammetry, Calcium Signaling, Accumbens, Striatum, Dopamine Transporter
Introduction
The mesolimbic dopamine (DA) system is heavily involved in both the therapeutic (e.g. for attention deficit hyperactivity disorder) and addictive properties of methamphetamine (METH). Methamphetamine has multiple effects on DA transmission, transiently increasing vesicular release, but also impairing DA uptake and vesicular packaging, resulting in intracellular DA accumulation and eventually DA efflux through the DA transporter (DAT; Chu et al. 2008; Fleckenstein et al. 2009; Hedges et al. 2018; Siciliano et al. 2014). The mechanisms underlying METH effects on DA transmission are incompletely characterized. Acutely, METH increase intracellular Ca2+ levels (Uramura et al. 2000; Yu et al. 2016), an effect mediated largely by voltage-gated Ca2+ channels (Yu et al. 2016), and partially by intracellular Ca2+ release (Yu et al. 2016). In cultured DA neurons, METH-induced increases in intracellular Ca2+ levels are attenuated by DAT blockade (Sambo et al. 2017). Since METH is a substrate for the DAT (Solis 2017), this previous finding suggests that in DA neurons, METH increases intracellular Ca2+ through interactions with intracellular machinery.
One target of interest for METH’s intracellular actions is the sigma receptor. The sigma receptor is a chaperone protein that induces intracellular Ca2+ release through the inositol 1,4,5-trisphosphate receptor (IP3R) (Fukunaga et al. 2015; Katz et al. 2011; Matsumoto et al. 2014; Nguyen et al. 2014; Su et al. 2010). Recently, we demonstrated that METH’s excitatory effects on vesicular DA signals and non-vesicular DA efflux are both reduced during sigma-receptor blockade (Hedges et al. 2018). Since sigma receptors are localized at the mitochondrial interface of the endoplasmic reticulum, IP3R activation may also result in increased Ca2+ release into the mitochondria, and subsequent increases in reactive oxygen species (ROS; Hedges et al. 2018). Increased ROS formation may have downstream effects on DA release and packaging machinery (Hedges et al. 2018) that result in elevated DA signals. However, little is known about the effects of IP3R activity on Ca2+ release in DA terminals and subsequent effects on DA signals. It is also unknown if METH effects on vesicular and non-vesicular DA involve IP3R activity. Therefore, this study aimed to examine the contribution of intracellular and extracellular Ca2+ sources in METH effects on DA.
Methods
Animal Subjects
Male C57BL/6 mice (PND 30–120) and Wistar rats (PND 80–120; Fig 5 only; Male [n=7] and Female [n=6]) were bred and cared for in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. At weaning (PND 21), animals were housed on a reverse light/dark cycle (lights on from 8 PM to 8 AM) in groups of 2–5/cage and given ad libitum access to food and water. Experimental protocols were approved by the Brigham Young University Institutional Animal Care and Use Committee and Korea Institute of Toxicology according to NIH guidelines.
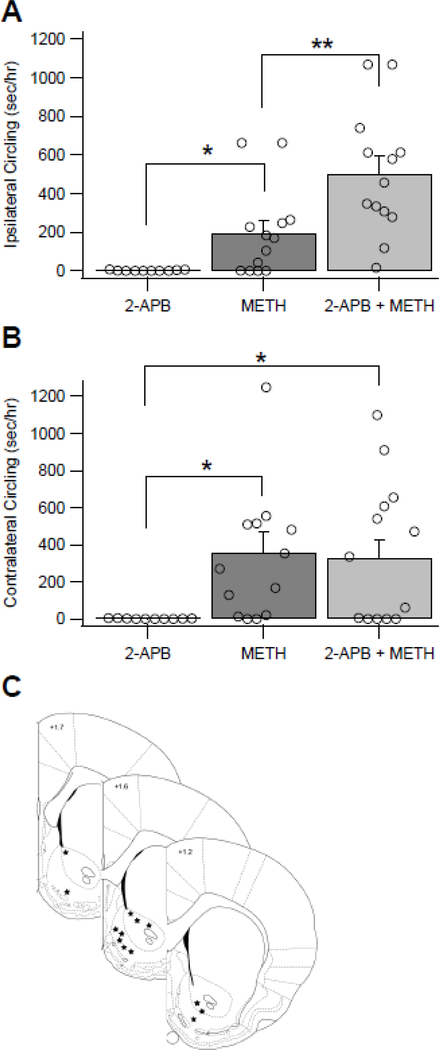
Figure 5.
Methamphetamine (METH)-induced Ca2+ release drives locomotor behavior. (A) Ipsilateral circling was increased following treatment with METH (5 mg/kg, IP) and 2-APB (0.5 μl @ 50 μM, intra-NAc) as compared to treatment with METH and vehicle. METH and vehicle also increased circling behavior relative to no METH. (B) Contralateral circling increased in METH and vehicle treated rats, as well as METH and 2-APB treated rats, compared to 2-APB treated controls. (C) Microinjection locations are shown for the 13 rats used in panel A. Stars indicate the location of the microinjections as determined following a dye injection and sectioning of the brain using a vibrating microtome. Asterisks (*) denote significance level p<0.05 and (**) denote significance level p<0.01.
Brain Slice Preparation
Coronal brain slices were obtained as previously described (Steffensen et al. 2008). Briefly, animals were anesthetized with isoflurane (5%), decapitated, and brains were rapidly dissected and sectioned into 400 μm slices in ice-cold artificial cerebrospinal fluid (ACSF) consisting of cutting solution consisting of (in mM): 220 Sucrose, 3 KCl, 1.25 NaH2PO4, 25 NaHCO3, 12 MgSO4, 10 Glucose, and 0.2 CaCl2. Slices were then transferred to room temperature artificial cerebrospinal fluid (ACSF) consisting of (in mM): 124 NaCl, 2 KCl, 1.25 NaH2PO4, 24 NaHCO3, 12 Glucose, 1.2 MgSO4, 2 CaCl2, pH 7.3, which was bubbled with 95% O2 / 5% CO2. The cutting solution consisted of (in mM): 220 Sucrose, 3 KCl, 1.25 NaH2PO4, 25 NaHCO3, 12 MgSO4, 10 Glucose, and 0.2 CaCl2. Slices were placed into an incubation chamber containing room temperature ACSF bubbled with carbogen. Slices were then transferred to a recording chamber with continuous ACSF flow (2.0 mL/min) maintained at 34–36 °C. The NAc was visualized at the level of the anterior commissure under low magnification with Nikon Diaphot inverted microscopes in the transmitted light mode and Olympus X51 microscopes with transmitted infrared Dodt gradient contrast imaging.
Fast Scan Cyclic Voltammetry Recordings
Two different measures of DA release were obtained using fast scan cyclic voltammetry: electrically evoked DA release (eDA), and DA efflux. For both release measures, carbon fiber electrodes (CFEs) were positioned at an angle, ~75 μm below the surface of the slice in the NAc core, localized to the anterior commissure. Dopamine release was electrically evoked every 2 min by biphasic stimulation (4 msec pulses, 10 pulse, 350 μA, 20 Hz) from an ACSF-filled micropipette tip broken to (5–10 μm tip diameter), filled with ACSF and placed 100–200 μm from the CFE. The CFE potential was linearly scanned from −0.4 to 1.2 V and back to −0.4 V vs Ag/AgCl (scan rate = 400 V/sec). Cyclic voltammograms were recorded every 100 msec (10 Hz) with ChemClamp potentiometers (Dagan Corporation, Minneapolis, MN, USA). Recordings were performed and analyzed using LabVIEW (National Instruments, Austin, TX, USA)-based Demon Voltammetry software (Yorgason et al. 2011a). Results were determined from maximum oxidation peak values for eDA experiments. The maximal amplitude of the eDA signal (peak nA value) was measured and analyzed for eDA experiments. This measurement does not use Michaelis-Menten kinetic modeling and thus includes components of release and uptake (Wightman and Zimmerman 1990; Yorgason et al. 2011a).
For DA efflux experiments, eDA signals were detected first to verify CFE placement. When eDA signals did not vary by more than 5% for five successive collections, the electrical stimulation was turned off and voltammograms were recorded at 0.5 Hz. Unless otherwise specified, all other recordings were performed with the pharmacological agent added at 30 min and METH at 60 min from the start of the recording. The voltammograms of long recordings with low METH concentrations were generally less clean than experiments with short recordings and high methamphetamine concentrations. Therefore, the latter was used to generate greater DA increases to better validate oxidation peaks in the voltammogram. Dopamine signals and DA efflux are expressed as a change in μM DA, not absolute extracellular DA concentration, due to the background-subtracted nature of voltammetric recordings.
Drug Preparation and Administration
Methamphetamine (Sigma-Aldrich; St. Louis, Missouri, USA), CdCl2 (Spectrum Chemical; Gardena, CA, USA), 1,2-Bis(2-aminophenoxy)ethane-N,N,N’,N’-tetraacetic acid tetrakis (acetoxymethyl ester) (BAPTA-AM; Cayman Chemical; Ann Arbor, MI, USA), cyclopiazonic acid (CPA; Cayman Chemical), 2-Aminoethoxydiphenylborane (2-APB; Tocris; Minneapolis, MN, USA), Thapsigargin (Tocris) were dissolved in stock solutions and then diluted into ACSF at specified concentrations.
Cell Culture and Differentiation of Human Neural Progenitor Cells (hNPCs)
Differentiation of hNPCs to DA-like neurons was previously described (Young et al. 2010). Briefly, hNPCs (Neuromics; Edina, MN, USA) were grown in dishes coated with Matrigel (Corning Inc; Corning, NY, USA) and cultured in 37°C and 5% CO2 conditions, in growth medium, consisting of AB2™ Basal Neural Media (ArunA Biomedical; Athens, GA, USA), 2mM L-alanyl-L-glutamine (HyClone Laboratories Inc; Logan, UT, USA), 1X Penicillin-Streptomycin (Gibco; Langley, OK, USA), 10 ng/mL LIF (PeproTech; Rocky Hill, NJ, USA), and 20ng/mL FGF-b (PeproTech). Media were replaced every 3–4 days, and cells were grown to 100% confluency before passaging. hNPCs were plated on poly-l-ornithine- and laminin-coated plates. Twenty-four hours after plating, hNPCs were then differentiated with growth medium (without FGF-b) with the addition of 25 ng/mL recombinant human GDNF (PeproTech). Fifty percent of cell media was removed every three to four days and replaced with fresh differentiating media containing glial cell-derived neurotrophic factor (GDNF).
Calcium Measurements
Differentiated cells or neurons were loaded with 1–5 μM Fura-2AM (Molecular Probes, CA) for 15 min at room temperature. Neurons plated on glass-bottom Petri-dishes were then placed on the microscope stage and perfused with Tyrode solution consisting of, in mM: 129 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 30 Glucose, and 25 HEPES for 3–4 minutes. A single experimental run consisted of 3–4 min perfusion with Tyrode solution followed by 20 min perfusion with 50 μM METH in Tyrode solution (or 50 μM METH in 0 external Ca2+ Tyrode solution or 50 μM methamphetamine + 10 μM thapsigargin in Tyrode solution). Calcium-free Tyrode solution consisted of, in mM: 129 NaCl, 5 KCl, 0 CaCl2, 3 MgCl2, 30 Glucose, and 25 HEPES. Ratiometric Ca2+ imaging was performed on neurons by illuminating them at 340 and 380 nm wavelengths at 10 sec intervals. Emitted light (above 490 nm) was then captured from neurons with an intensified CCD camera interfaced with an inverted microscope. Fluorescent intensities from cell images were analyzed using Winfluor Software (University of Strathclyde, Glasgow, Scotland). The Ca2+ level was expressed as the ratio of fluorescence intensities excited at 340nm /380nm (after background subtraction). Average fluorescent ratios were then calculated from the number of regions of interest (ROI) as indicated in the text.
Immunohistochemistry
hNPC cells were differentiated for 7 days or more. Then, they were fixed with 2% paraformaldehyde at room temperature for 10 minutes and washed three times with 1x PBS. Cells were then permeabilized with 1x PBST at room temperature for 10 minutes then blocked for 10 minutes with 1% normal goat serum in PBST. Cells were further washed, three more times, with PBST and then incubated with 1:200 dilution of dopamine transporter antibody (Millipore Sigma, MA) for 1 hour at room temperature. Cells were washed three more times before incubating with 1:1,000 dilution of goat anti-rabbit secondary antibody against Alexa Fluor 488 (Thermo Fisher Scientific, MA) at room temperature for 40 minutes. Three additional washes were done with 1x PBST, and then the cells were mounted with DAPI Fluoromount-G® (SouthernBiotech, AL).
Circling Behavior
Rats were implanted with a guide cannula (MD-2250, Basi Instruments, West Lafayette, IN) in the ventral striatum (+1.2 to +1.7 AP, −0.8 to −2.2 ML, +5.2 to +7.0 DV). The guide cannula was fixed in place using dental cement. Subjects were maintained under isoflurane anesthesia (1.5% - 2.0%) throughout the procedure and were allowed a minimum of 1 week of recovery time prior to experimentation. Circling experiments were performed in a 16” x 16” x 16” plexiglass compartment. On test days, the subjects were injected intraperitoneally (IP) with either 5 mg/kg METH dissolved at 5 mg/ml in 0.9% NaCl saline solution or 1 ml/kg 0.9% NaCl saline solution. This injection was followed 5 min later by either a 0.5 μl injection of 50 μm 2-APB dissolved in 0.05% DMSO in ACSF or a 0.5 μl injection of 0.05% DMSO in ACSF into the ventral striatum. Microinjections were accomplished using a microinjection pump (A-99, Razel Scientific Instruments, Fairfax, VT) and a 25 μl glass syringe (Hamilton Robotics, Reno, NV) connected by Silastic tubing to an injection needle that extended 1.0 mm past the end of the guide cannula. The injection occurred over 30 seconds, with the needle left in place for an additional 30 sec prior to removal. Subjects were briefly and lightly anesthetized with Isoflurane (4.0%) for all injections in order to avoid the stress of the injection in subjects with chronic implants and repeated measures. Following the injections, subjects were placed in the behavioral apparatus for 1 hr and their locomotor activity was recorded using a camera mounted on the ceiling above the apparatus connected to a Windows 7 PC running Pinnacle Studio 16 (Corel Inc., Menlo Park, CA). The videos were manually scored for general locomotor activity and time spent circling. Injection order was counterbalanced across rats to avoid order effects. At the end of the experiments, rats were injected with 0.5 μl of 100 mM pontamine sky blue (Avocado Research Chemicals Limited, Lancashire, United Kingdom) dissolved in distilled water through the cannula. Rats were then euthanized, and brains were removed for sectioning using a vibrating microtome. Sectioned brain slices were then examined to determine the location of the microinjection.
Statistical Analyses
The eDA signals were measured (in nA) throughout the experiment. Methamphetamine effects followed the time course previously reported (Hedges et al. 2018), with peak effects occurring in the first ~4–6 minutes, and stability reached after 50–60 minutes of drug exposure. Therefore, the peak effects of METH (maximal responses during the first 10 minutes of drug application) are the reported values for the data reported and traces shown in this study. Baseline (pre-drug/pre-METH) measurements of eDA were obtained after signals reached stability (three collections with ≤10% variability). The average amplitude of the three baseline signals was calculated, and subsequent peak nA values were divided by the baseline average to produce a normalized response. Thus, all eDA signals (traces and bar plots) are reported as values normalized to pre-drug/pre-METH within subject controls.
For DA efflux experiments, the signal evoked by METH generally returned to baseline after 30–60 min (Hedges et al. 2018). Therefore, the area under the curve (AUC) between the initial DA increase and the return to baseline was calculated using Igor Pro 7.0 across a similar time frame (WaveMetrics; Lake Oswego, OR, USA). The AUC was then divided by the change in time of the DA efflux as previously described (Hedges et al., 2018), standardizing experiments for average DA detected per sec. Drug perfusion, data acquisition and analysis were performed for the same amount of time between groups. Thus, experiments controlled for the amount of time a brain slice was drug exposed. Values were grand-averaged across animals. Values are expressed as means ± SEM for cumulated data.
For locomotor behavior (general locomotor and circling), each animal was exposed to a specific drug condition for 5 trials, and the median value across these 5 trials was analyzed and plotted. Thus, each data point in behavioral results represents a median response from a single animal. For general locomotor activity, data is expressed as percent baseline locomotor activity. Circling behavior is expressed as seconds spent circling across the 1 hour period after the injection ([s/hr]). Two animals were excluded from the study for consistently displaying decreased locomotor activity following METH administration since changes in circling behavior are contingent upon an observed behavioral response. Statistics were performed using IBM SPSS Statistics 21 (Armonk, NY, USA) or STATA 15.1 (College Station, TX, USA). A two-tailed Student’s t test was used for comparisons with only two variables. To determine significance between more than two variables, a one-way analysis of variance (ANOVA) was used with TUKEY’s posthoc analysis. For all experiments, the criterion of significance was set at p < 0.05 (*), p < 0.01 (**), and p < 0.001 (***).
Results
Methamphetamine Induces Increases in Intracellular Calcium in Cultured Dopamine-like Cells
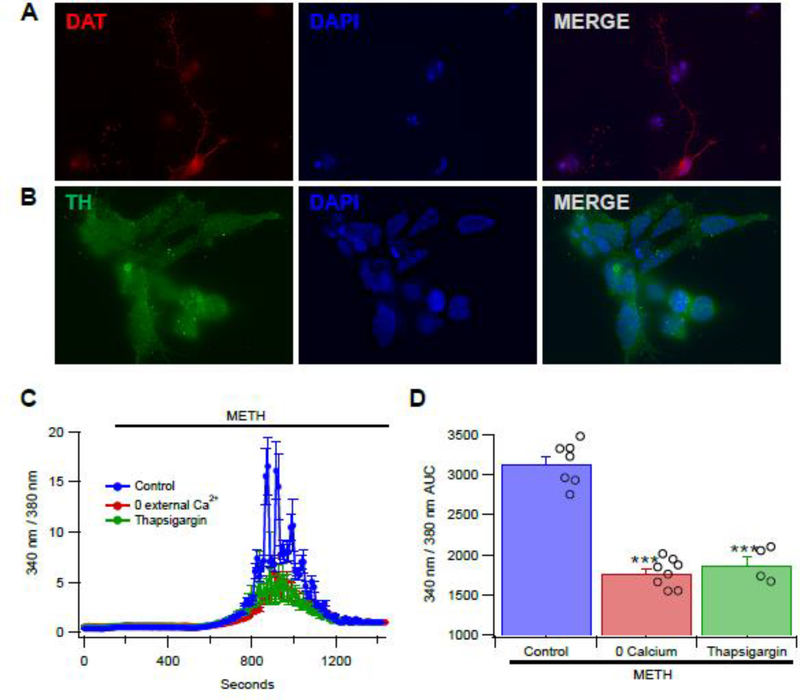
The effects of METH (50 μM) on intracellular Ca2+ release was tested in hNPC-derived, DA-like cells (Young et al. 2010). These cells are similar to DA neurons in that they express DAT and vesicular monoamine transporter 2 (VMAT) proteins (Young et al. 2010). Consistent with these previous findings, differentiated hNPCs in this study also exhibited the expression of DAT (Fig. 1A) and tyrosine hydroxylase (Fig. 1B). Perfusion of METH produced a robust increase in intracellular Ca2+ levels, reaching peak levels in 10 min and gradually decaying to baseline levels 16 min after the initial application of METH (Fig. 1C). Omitting Ca2+ from the external medium resulted in METH-evoked Ca2+ responses that were significantly reduced by >50%. Similarly, depletion of intracellular stores by thapsigargin (10 μM), an inhibitor of sarco/endoplasmic reticulum Ca2+ ATPase (SERCA), also reduced METH responses by the same magnitude (>50%). Subsequent analyses of the area under the Ca2+ levels-time curve (340/380 AUC- time) in Figure 1A show that METH responses were significantly attenuated by 0 external Ca2+ conditions (F(1,16) = 143.97, p < 0.0001) as well as by the co-application of thapsigargin with METH (F(1,16) = 82.51, p < 0.0001; Fig. 1D).
Figure 1.
Methamphetamine (METH) releases intracellular calcium in hNPC-derived, DA-like cells. (A) a Day-10 differentiated hNPC that is immune-positive for DAT (red, top left panel) and (B) Day-14 differentiated hNPCs that are immune-positive for tyrosine hydroxylase (green, bottom left panel). DAPI stains, to visualize the nuclei, are shown in the middle panels; and, merged images are in the right panels. (C) Perfusing METH (50 μM) increases Ca2+. Peak release occurs approximately 10 min after METH administration and subsides approximately 16 min after METH perfusion. Perfusing METH in the presence of zero Ca2+, decreases the amount of METH induced Ca2+ release by about 50%. Thapsigargin also reduces the amount of Ca2+ released by METH by about 50%. (D) Summary plot comparing Ca2+ release induced by METH. Asterisks (***) represent significance level p<0.001.
Reducing Intracellular Calcium Attenuates Methamphetamine-induced Increases in Dopamine in the Nucleus Accumbens
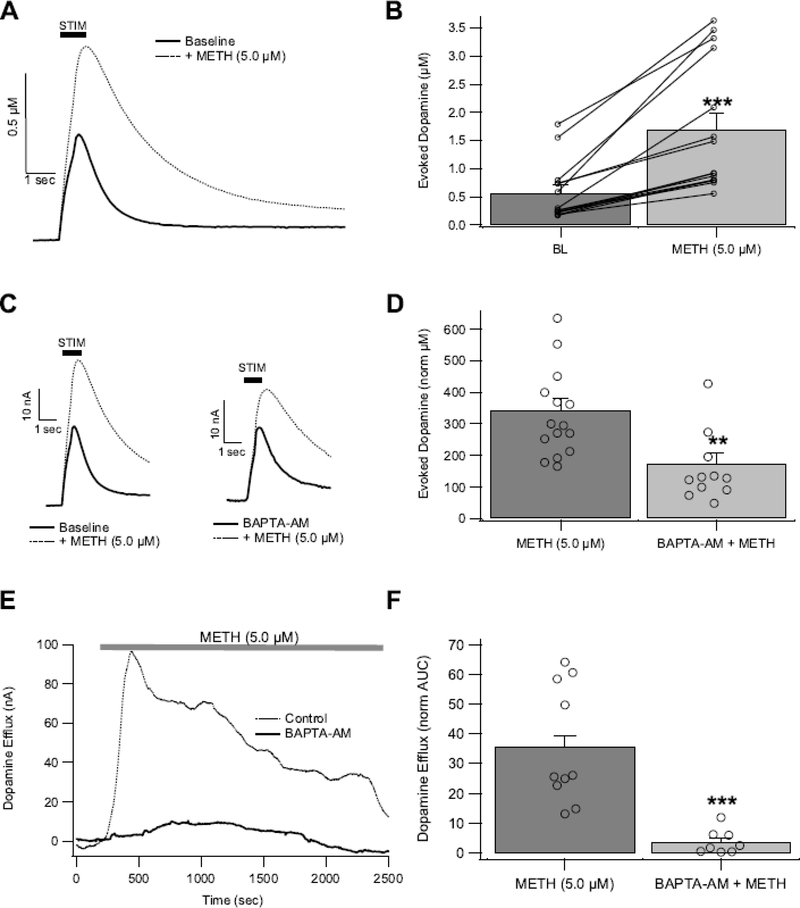
As we have demonstrated previously (Hedges et al. 2018), METH (5 μM) significantly increased eDA in the NAc, (t(14) = 5.5, p = 7.9E-05; Fig. 2A,B) with peak effects occurring at ~4–6 min (Hedges et al. 2018). The effects of intracellular Ca2+ chelation on eDA and METH interactions was tested. Intracellular rapid Ca2+ chelation was performed by bath application of the membrane permeable Ca2+ chelator BAPTA-AM (0.5 μM). Importantly, BAPTA-AM is only active intracellularly where it is trapped and activated after de-esterification (Tsien 1981). BAPTA-AM reduced eDA to 82 ± 0.6% (t(20) = 3.188, p = 0.0046), and attenuated METH-induced increases in eDA (Fig. 2C,D; t(24) = 3.53, p = 0.002). Methamphetamine also induced non-vesicular DA efflux via reverse transport of the DAT (Fig. 2E), with maximal effects at 5–10 μM (Hedges et al. 2018) and no apparent difference between these two concentrations (5 μM: 43.3 ± 11 norm AUC; 10 μM: 31.1 ± 7.6 norm AUC; t(8) = 0.9477; p = 0.371). These METH efflux effects were collapsed across concentrations for comparisons in other DA efflux experiments. In the presence of BAPTA-AM, METH-induced DA efflux was attenuated (Fig. 2E,F; t(17) = 4.836, p = 0.0002). Therefore, rapid and selective chelation of intracellular Ca2+ reduces the effects of METH on vesicular eDA and non-vesicular DA efflux.
Figure 2.
Chelating intracellular Ca2+ decreases METH-induced DA release in the NAc. (A,B) Methamphetamine (5 μM) acutely increases electrically-evoked DA (eDA) signals. Pretreating tissue with BAPTA-AM decreases METH-induced eDA (C,D) and non-electrically stimulated DA efflux (E,F). Asterisks (**, and ***) denote significance levels p<0.01, and p<0.001, respectively. Open circles indicate individual values.
Reducing Extracellular Calcium Attenuates Methamphetamine-induced Dopamine Efflux in the Nucleus Accumbens
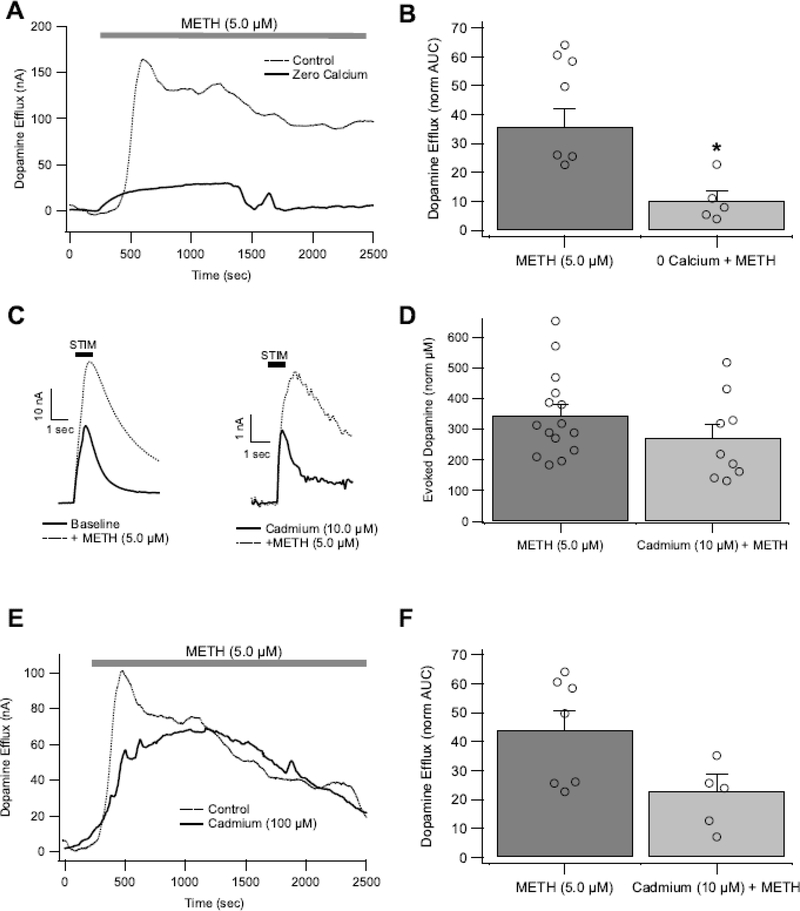
Next, the effects of extracellular Ca2+ removal was tested. Under zero Ca2+conditions, eDA was abolished (data not shown). Therefore, the effects of zero extracellular Ca2+ on METH effects were restricted to DA efflux measures. Under zero Ca2+ conditions, METH-induced DA efflux still occurred, though was markedly reduced (Fig. 3A,B; t(13 )= 2.757, p = 0.0163). Therefore, METH-induced efflux is partly dependent upon extracellular Ca2+ levels, possibly through voltage-gated Ca2+ channels (CaVs). Thus, the role of CaVs in METH effects on eDA and DA efflux was tested. During electrical stimulation, CaVs are recruited, resulting in Ca2+ influx that triggers vesicular fusion (Brimblecombe et al. 2015; Sudhof 2004). The non-selective CaV blocker Cd2+ was applied at low and high concentrations (10, 100 μM). At high concentrations, Cd2+ abolished eDA (data not shown), but at low concentrations Cd2+ (10 μM) reduced eDA to 24.6 ± 6% (t(12) = 5.05, p < 0.0003). Methamphetamine (5 μM) enhanced eDA under Cd2+ (10 μM) conditions to a similar extent as untreated controls (Fig. 3C,D; t(,15) = 0.96, p = 0.3502). Thus, voltage-gated Ca2+ channels do not appear to play a major role in METH effects on eDA signals. As Cd2+ at higher concentrations (100 μM) abolished eDA, this maximal concentration of Cd2+ was used only for DA efflux experiments. Cadmium (100 μM) did not block METH-induced DA efflux (Fig. 3E,F; t(12) = 1.626, p = 0.1299), despite the slight trend for reduced effects. Considering the weak effects of Cd2+ on METH-induced DA increases (both for eDA and DA efflux), voltage-gated Ca2+ channels do not appear to play a major role in METH effects on DA in the NAc. In combination with the zero Ca2+ data, these data suggest that extracellular Ca2+ may affect METH efflux by disrupting intracellular Ca2+ signaling in general and may even involve other non-voltage-gated Ca2+ channels. Furthermore, since Cd2+and zero Ca2+ both blocked action-potential-dependent eDA, but only zero Ca2+ conditions reduced METH-induced DA efflux, action potentials are not required for efflux to occur. This conclusion is also supported by our previous study where lidocaine blocked action potential-dependent eDA signals but not METH-induced DA efflux (Hedges et al. 2018).
Figure 3.
Extracellular Ca2+ drives METH-induced DA efflux in the NAc, but is not critical for METH-induced DA efflux or increases in electrically evoked DA (eDA). (A,B) Decreasing the amount of Ca2+ in extracellular fluid attenuates METH-induced DA efflux. Blocking CaV channels with Cd2+ did not significantly affect METH-induced increases in eDA (C,D) or DA efflux (E,F). Asterisk (*) denotes significance level p<0.05. Open circles indicate individual values.
Methamphetamine-induced Increases in Dopamine Involve Intracellular Calcium Stores
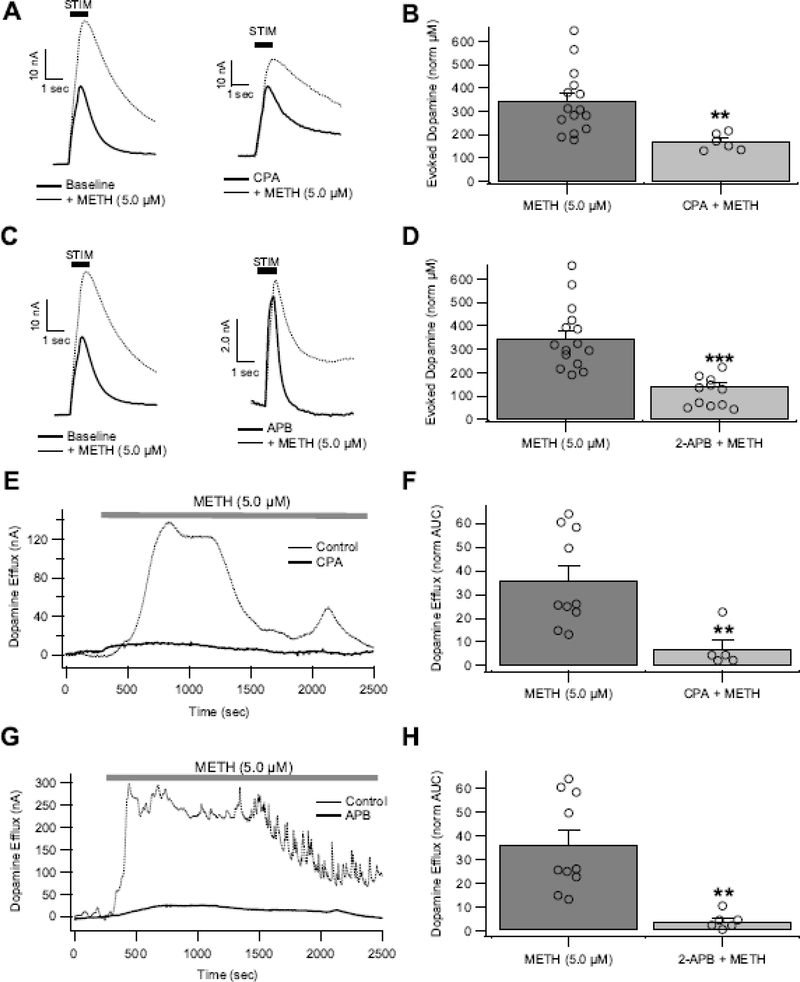
Calcium release from endoplasmic reticulum intracellular stores are a major source of Ca2+ signaling. It is unknown how intracellular Ca2+ stores contribute to DA transmission or METH effects on DA transmission. Therefore, two pharmacological approaches were taken to investigate the role of intracellular Ca2+ stores in METH effects on DA. First, the effects of intracellular Ca2+ depletion was tested by blocking the SERCA with cyclopiazonic acid (CPA), which is similar to thapsigargin (Fig. 1), in that both drugs block SERCA, resulting in impaired Ca2+ storage and subsequent store depletion (Bhogal and Colyer 1998; Dettbarn and Palade 1998; Seidler et al. 1989). Second, the effects of blocking Ca2+ release through IP3R channels was tested using 2-aminoethoxydiphenylborane (2-APB). Importantly, 2-APB specifically inhibits Ca2+ release from IP3Rs, but does not affect Ca2+ packaging via the SERCA at concentrations used in this study (minimal inhibition observed at concentrations <200 μM) (Bilmen et al. 2002). Despite different pharmacological mechanisms, the effects of these two drugs were compared. Bath application of CPA (10 μM) and 2-APB (50 μM) had no effect on eDA (CPA: 90.7 ± 10%, t(7) = 0.93, p = 0.386; 2-APB: 96.88 ±10.1%, t(17) = 0.29, p = 0.77; paired two-tailed t test) in the absence of METH. Therefore, intracellular Ca2+ stores are not recruited during eDA for baseline conditions. Methamphetamine-induced increases in eDA were significantly reduced by CPA (t(18) = 4.5, p = 0.0003; Fig. 4A,B) and abolished by 2-APB (t(1,21) = 5.9, p = 7.3E-06; Fig. 4C,D), suggesting that METH is acting via intracellular Ca2+ stores to facilitate vesicular eDA. Next, the role of intracellular stores in non-vesicular DA efflux were evaluated. Methamphetamine-induced DA efflux in CPA and 2-APB treated slices was reduced compared to non-CPA/2-APB pretreated slices (Fig. 4E–H; t(13) = 3.05, p = 0.0093; 2-APB: t(14) = 3.847, p = 0.0018). Therefore, intracellular Ca2+ stores are recruited in the presence of METH, resulting in increased vesicular and non-vesicular DA, likely through sigma-receptor interactions (Hedges et al. 2018).
Figure 4.
Intracellular Ca2+ signaling drives METH-induced increases in DA release in the NAc. Pretreatment with CPA (10 μM; A,B) and 2-APB (50 μM; C,D) attenuated METH-induced increases in electrically stimulated DA (eDA). Pretreatment with CPA (10 μM; E,F) reduced and 2-APB (50 μM; G,H) prevented METH-induced DA efflux. Asterisk (**, ***) denote significance levels p<0.01 and p<0.001, respectively. Open circles indicate individual values.
Methamphetamine-induced Calcium Release is Involved in Dopamine-related Locomotor Behavior
Since METH increases general locomotor activity via DA-dependent processes, impairing METH effects on DA transmission on one side of the brain produces ipsilateral circling behavior in rats (Hedges et al. 2018; Jalewa et al. 2017; Saigusa et al. 1993). Therefore, to assess the behavioral relevance of intracellular Ca2+ release in METH effects on DA transmission, METH-induced (5 mg/kg, IP) increases in ipsilateral circling behavior were evaluated in rats after intra-NAc infusions of 2-APB across a 1 hr period. Generalized locomotor activity increased following systemic METH (5 mg/kg, IP) administration (t(12) = 3.305, p = 0.003, one-tailed paired t-test; METH + vehicle: 227.47 ± 32.23%, n = 13; No METH (i.e. IP saline): 95.50 ± 11.21%, n = 13). General locomotor increases were similar in both METH + vehicle and METH + 2-APB animals (t(12) = −1.286, p= 0.222, two-tailed paired t-test; METH + vehicle: 227.47 ± 32.23%, n = 13; METH + 2-APB: 266.41 ± 47.66%, n = 13). Next, changes in circling behavior were examined (reported as time spent circling in seconds/hour [s/hr]). Ipsilateral circling was significantly increased by systemic METH administration (Fig. 5A; t(9) = 2.257, p = 0.025, one-tailed paired t-test; No METH + 2-APB: 1.81 ± 0.89 [s/hr], n = 10; METH + vehicle: 190.26 ± 83.09 [s/hr], n = 10). Ipsilateral circling was further increased by systemic METH when 2-APB was infused in the NAc, as compared with systemic METH when vehicle was infused in the NAc (Fig. 5A; t(12) = −3.314, p = 0.006, two-tailed; METH + 2-APB: 502.03 ± 89.50 [s/hr], n = 13; METH + vehicle: 197.20 ± 63.44 [s/hr], n = 13). Contralateral circling was significantly increased by systemic METH administration (Fig. 5B; t(9)= 2.179, p= 0.029, one-tailed paired t-test; No METH + 2-APB: 1.46 ± 0.60 [s/hr], n = 10; METH + vehicle: 257.62 ± 117.72 [s/hr], n = 9), however no further increase in contralateral circling was observed following systemic METH when 2-APB was infused in the NAc (Fig. 5B; t(12) = 0.586, p = 0.569, two-tailed paired t-test; METH + 2-APB: 327.65 ± 96.97 [s/hr], n = 13; METH + vehicle: 359.86 ± 106.58 [s/hr], n = 13). Cannula placements in the NAc were histologically confirmed after circling experiments (Fig. 5C). Thus, intra-accumbal perfusion of 2-APB, and subsequent effects on intracellular Ca2+ signaling, disrupt METH-induced locomotor behavior to produce increases in ipsilateral circling.
Discussion
This study examined the role of Ca2+ in METH-induced increases in vesicular and non-vesicular DA signals. The results indicate that METH induces a transient increase in intracellular Ca2+ that is attenuated by IP3 receptor blockers and in the absence of extracellular Ca2+. Pharmacological manipulations that reduce intracellular Ca2+ in striatal slices also attenuated METH effects on DA. Reducing extracellular Ca2+ also reduced METH-induced DA efflux. Blocking voltage-gated Ca2+ channels does not appear to change METH effects on stimulated or non-stimulated DA. Methamphetamine-induced circling behavior was increased with intra-NAc 2-APB, demonstrating a role for intracellular Ca2+ on reinforcement-related behaviors.
Extracellular Calcium and Methamphetamine
In the present study, the zero extracellular Ca2+ conditions attenuated METH-induced intracellular Ca2+ increases and prevented DA efflux, suggesting that extracellular Ca2+ influx is involved in METH effects on non-vesicular DA release. The role of extracellular Ca2+ in this effect is still somewhat ambiguous. However, since blocking voltage-gated Ca2+ channels reduced eDA signals but did not reduce effects of METH on eDA, the mechanisms involved in these two METH effects are differentially sensitive to extracellular Ca2+. A previous study indicated that METH induces increases in intracellular Ca2+ in cortical neurons, an effect that is blocked by MK801, Mg2+, low Ca2+ and L-type blockers (Yu et al. 2016). Similarly, in isolated DA neurons, METH caused Ca2+ oscillations that were sensitive to low Ca2+, as well as N-type (ω-conotoxin), P/Q-type (ω-agatoxin), L-type (nitrendipine) and T-type (Nickel) Ca2+ channel blockers (Uramura et al. 2000). Together, these previous studies indicate that voltage-gated Ca2+ channels and ionotropic glutamate receptors are somehow involved in METH effects on intracellular Ca2+ influx at cell bodies. Yu et al also tested the role of ER sources of Ca2+ release in METH effects and found that blocking ER Ca2+release with the ryanodine receptor antagonist dantrolene only partially blocked METH effects compared to application of Mg2+ and Ca2+ channel blockers, suggesting that METH-enhanced Ca2+ influx may influence intracellular Ca2+ stores to produce downstream effects. Another study showed increased Ca2+ via L-type Ca2+ channels in a heterologous expression system (Sugimoto et al. 2009). Taken together, METH increases intracellular Ca2+ through multiple pathways that may depend on cell type, as well as location within the cell (e.g. soma vs terminals). It was shown previously that T- and L-type Ca2+ channels are minimally involved in DA release in the NAc, and instead N and P/Q type channels predominate under normal Ca2+ conditions (Brimblecombe et al. 2015). This is in contrast to the dorsal striatum where T- and L-type blockers reduce eDA to 60–70% of control values in voltammetry studies (Brimblecombe et al. 2015) and K+ evoked DA release is attenuated by L-blockers in microdialysis (Zhu et al. 2004). Considering this regional difference in Ca2+ channel usage between the NAc and dorsal striatum, and previous studies showing METH effects through voltage-gated Ca2+ channels, it would be interesting to know if METH effects in the dorsal striatum involve Ca2+ influx through T/L type channels. Since voltage-gated Ca2+ channels are depolarization-dependent, it is remarkable that METH-induced efflux is somewhat attenuated by Cd2+ under non-electrically stimulated conditions, suggesting that spontaneous depolarizations at terminals (in particular during in vivo conditions; Yorgason et al. 2017) may be important for efflux effects. Lastly, it should be noted that acute METH inhibits L- and N-type channels, albeit at supraphysiological concentrations (1 mM; Andres et al. 2015).
Intracellular Calcium Stores and Methamphetamine
Intracellular Ca2+ stores play an important role in DA activity at the cell body (Cui et al. 2004; Kramer and Williams 2016), but not much is known about how intracellular stores affect release at terminals. Microdialysis studies have shown that K+ evoked DA release in the striatum is reduced by IP3R antagonists and enhanced by IP3 agonists (Zhu et al. 2004). In contrast, ryanodine receptor antagonists do not affect K+ stimulated DA release, indicating a preference for IP3R activity in DA terminals under these stimulation conditions (Zhu et al. 2004). Here, the SERCA inhibitor CPA, and the IP3R antagonist 2-APB were applied to NAc slices during voltammetric recordings of eDA signals. While neither of these drugs significantly reduced eDA on their own, both drugs were effective at reducing METH’s effects on eDA signals. Since these drugs had no effect on their own, this suggests that under ‘normal’ recording conditions, intracellular Ca2+ release through the IP3R, and subsequent effects on DA terminals, is minimal. Furthermore, since METH effects are sensitive to these blockers, METH appears to be enhancing IP3R activity. This is consonant with the what we observed in DA-like derived hNPC cells where IP3R antagonism with thapsigargin prevented METH effects on intracellular Ca2+ levels (Fig. 1). We have previously demonstrated that blocking sigma 1 receptors also reduces METH enhancement of eDA and DA efflux (Hedges et al. 2018). This is fundamental to the current study because sigma 1 receptors are located on intracellular endoplasmic reticulum domains, and when activated they initiate Ca2+ release through IP3Rs (Ruscher and Wieloch 2015). Since METH is a potent sigma receptor agonist (Maurice and Su 2009), it appears that its effects on intracellular Ca2+ release are secondary to effects on other intracellular machinery. It should be emphasized that eDA signals contain components of DA release and uptake (Wightman and Zimmerman 1990; Yorgason et al. 2011a) that were not examined independently in the present study. Changes in eDA amplitude induced by METH may reflect changes in uptake or release machinery (both Ca2+ sensitive and insensitive). Previously, it was shown that amphetamine induced increases in DA release were not present in DAT-KO animals (Siciliano et al. 2014), suggesting that the DAT is required for some aspects of METH-induced increases in eDA. Presently, reducing intracellular Ca2+ resulted in attenuated METH effects on eDA. In light of the previous DAT-KO study (Siciliano et al. 2014), METH-induced increases in intracellular Ca2+ and downstream effects on DA transmission may involve DAT specific interactions. However, an important consideration is that the Siciliano et al study only reported effects of amphetamine after eDA stabilized (typically ~40 minutes), and not a full time course (Siciliano et al. 2014). Contrariwise, METH (5 μM) enhances eDA within the first ~4–6 minutes, which didn’t reach “stability” until ~50 minutes after drug application (Hedges et al. 2018). Therefore, the DAT-dependent amphetamine-induced increases in eDA observed by Siciliano et al may involve other processes that are recruited after a long application. Importantly, the DA enhancing psychostimulant effects at the DAT (Yorgason et al. 2011b) and subjective effects in humans for the “high” associated with psychostimulants are rapid, occurring within the first few seconds (Evans et al. 1996; Seecof and Tennant 1986; Zernig et al. 2003) and peaking within 10–15 minutes (Newton et al. 2005). Therefore, future studies examining the role of the DAT in amphetamine/METH induced changes in eDA would benefit from examining peak effects at these early time points as well as later time points where homeostatic mechanisms have stabilized the signal.
Since eDA signals represent changes in release and uptake, METH likely increases DA release/detection through multiple mechanisms. Furthermore, some of the enhancing effects of METH through the IP3R may be masked in part through D2 autoreceptor activation and subsequent decreases in exocytotic release (Branch and Beckstead 2012; Ford 2014). Therefore, since METH-induced increases in eDA are evident despite D2 autoreceptor activation, this suggests that IP3R activity is sufficient to overcome D2 autoinhibition. Our sigma receptor study demonstrated that METH effects through the sigma receptor involved increased reactive oxygen species (ROS), likely through enhanced Ca2+ signaling into mitochondria (Hedges et al. 2018; Ruscher and Wieloch 2015). Enhanced ROS in DA terminals resulted in VMAT glutathionylation and subsequent impairment of vesicular packaging (Hedges et al. 2018). These changes in VMAT activity help explain the increased “leakiness” of DA vesicles, and subsequent DA efflux. Thus, the present studies corroborate these previous findings by demonstrating a role for intracellular Ca2+ signaling in efflux experiments.
Methamphetamine-induced Locomotion and Intracellular Calcium Interactions with Circling Behavior
Methamphetamine increases locomotor activity and circling in a DA-dependent manner (Jang et al. 2017). Furthermore, unilateral 6-OHDA lesions of the mesolimbic pathway decrease endogenous DA levels and DA release (Kelly et al. 1975; Wasik et al. 2018), and produce ipsilateral circling behavior in rats (Ali et al. 1995; Jalewa et al. 2017). Unilateral NAc injections with D1/D2 receptor agonists, which is thought to mimic increased DA levels, can induce contralateral circling, an effect abolished by ipsilateral D1/D2 receptor antagonists (Ikeda et al. 2007; Saigusa et al. 1993). Therefore, METH-induced circling results from an imbalance in DA transmission between the two hemispheres. In this study, the IP3R inhibitor 2-APB was effective at preventing METH-induced increases in DA release (Fig. 4). Also, unilateral injections of 2-APB in the NAc produced a significant increase in ipsilateral circling behavior following treatment with systemic METH. This increase was larger in magnitude than that produced by METH when vehicle was injected in the NAc. The increase in circling produced by METH alone was not specific to ipsilateral circling as contralateral circling was also increased, suggesting that the increase in circling was related to generalized locomotor activity. Unilateral injections of 2-APB in the NAc followed by METH produced only an increase in ipsilateral circling and not contralateral circling as compared with METH and vehicle co-administration. Therefore, 2-APB decreases the magnitude of METH-induced DA release in vivo thus causing an imbalance in NAc DA transmission and a subsequent increase in ipsilateral circling behavior. Thus, IP3R Ca2+ signaling plays a key role in METH’s ability to increase DA release and cause behavioral changes. We previously demonstrated that unilateral intra-NAc sigma receptor antagonists similarly induce circling behavior (Hedges et al. 2018). Although sigma receptors were not tested in the current study, in our proposed model, METH activates sigma receptors to induce Ca2+ release through IP3Rs, resulting in downstream effects on DA release (Hedges et al. 2018). Therefore, these two studies provide evidence for two separate, but related, mechanisms that may be effective targets for reducing psychostimulant reinforcement and seeking behavior.
In conclusion, METH increases vesicular and non-vesicular DA signals through multiple mechanisms. Methamphetamine increases DA release/levels in part through impaired DA clearance, but also through enhanced intracellular Ca2+ release and downstream signaling on release and packaging machinery. Impaired vesicular packaging through ROS-induced modification of VMAT is involved in efflux effects. Intracellular Ca2+ release and downstream effects on readily releasable pools are involved in the excitatory effects of METH on vesicular release. Notably, intra-NAc blockade of intracellular Ca2+ release produced increases in circling behavior, supporting a role for METH-induced IP3R activity in DA-related behaviors. Elucidating the downstream effects of Ca2+ on intracellular stores on readily releasable pools will provide useful formation for the abuse and therapeutic effects of METH on DA release.
Acknowledgements
We would like to acknowledge Chris Schow, Gilbert Marchant, Spencer McCarthy, Seth Stapley, Austin Elwood, Sadie Karratti-Abordo and Chena Bryan for technical assistance during experiments. We would like to acknowledge funding from the National Research Foundation of Korea (NRF; 2016R1D1A1B03935206 to EYJ) and National Institute of Drug Abuse (NIDA; R01DA035958 to SCS and R03DA033904 to MAA). The authors have no competing conflicts of interest to disclose.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- Ali SF, Kordsmeier KJ, Gough B (1995) Drug-induced circling preference in rats. Correlation with monoamine levels Mol Neurobiol 11:145–154 doi: 10.1007/BF02740691 [DOI] [PubMed] [Google Scholar]
- Andres MA et al. (2015) Methamphetamine acutely inhibits voltage-gated calcium channels but chronically up-regulates L-type channels J Neurochem 134:56–65 doi: 10.1111/jnc.13104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhogal MS, Colyer J (1998) Depletion of Ca2+ from the sarcoplasmic reticulum of cardiac muscle prompts phosphorylation of phospholamban to stimulate store refilling Proc Natl Acad Sci U S A 95:1484–1489 doi: 10.1073/pnas.95.4.1484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilmen JG, Wootton LL, Godfrey RE, Smart OS, Michelangeli F (2002) Inhibition of SERCA Ca2+ pumps by 2-aminoethoxydiphenyl borate (2-APB). 2-APB reduces both Ca2+ binding and phosphoryl transfer from ATP, by interfering with the pathway leading to the Ca2+-binding sites Eur J Biochem 269:3678–3687 doi: 10.1046/j.1432-1033.2002.03060.x [DOI] [PubMed] [Google Scholar]
- Branch SY, Beckstead MJ (2012) Methamphetamine produces bidirectional, concentration-dependent effects on dopamine neuron excitability and dopamine-mediated synaptic currents J Neurophysiol 108:802–809 doi: 10.1152/jn.00094.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brimblecombe KR, Gracie CJ, Platt NJ, Cragg SJ (2015) Gating of dopamine transmission by calcium and axonal N-, Q-, T- and L-type voltage-gated calcium channels differs between striatal domains J Physiol 593:929–946 doi: 10.1113/jphysiol.2014.285890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu PW et al. (2008) Differential regional effects of methamphetamine on dopamine transport Eur J Pharmacol 590:105–110 doi: 10.1016/j.ejphar.2008.05.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui G, Okamoto T, Morikawa H (2004) Spontaneous opening of T-type Ca2+ channels contributes to the irregular firing of dopamine neurons in neonatal rats J Neurosci 24:11079–11087 doi: 10.1523/JNEUROSCI.2713-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dettbarn C, Palade P (1998) Effects of three sarcoplasmic/endoplasmic reticulum Ca++ pump inhibitors on release channels of intracellular stores J Pharmacol Exp Ther 285:739–745 [PubMed] [Google Scholar]
- Evans SM, Cone EJ, Henningfield JE (1996) Arterial and venous cocaine plasma concentrations in humans: relationship to route of administration, cardiovascular effects and subjective effects J Pharmacol Exp Ther 279:1345–1356 [PubMed] [Google Scholar]
- Fleckenstein AE, Volz TJ, Hanson GR (2009) Psychostimulant-induced alterations in vesicular monoamine transporter-2 function: neurotoxic and therapeutic implications Neuropharmacology 56 Suppl 1:133–138 doi: 10.1016/j.neuropharm.2008.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford CP (2014) The role of D2-autoreceptors in regulating dopamine neuron activity and transmission Neuroscience doi: 10.1016/j.neuroscience.2014.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukunaga K, Shinoda Y, Tagashira H (2015) The role of SIGMAR1 gene mutation and mitochondrial dysfunction in amyotrophic lateral sclerosis J Pharmacol Sci 127:36–41 doi: 10.1016/j.jphs.2014.12.012 [DOI] [PubMed] [Google Scholar]
- Hedges DM et al. (2018) Methamphetamine Induces Dopamine Release in the Nucleus Accumbens Through a Sigma Receptor-Mediated Pathway Neuropsychopharmacology 43:1405–1414 doi: 10.1038/npp.2017.291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda H, Kotani A, Koshikawa N, Cools AR (2007) A vehicle injection into the right core of the nucleus accumbens both reverses the region-specificity and alters the type of contralateral turning elicited by unilateral stimulation of dopamine D2/D3 and D1 receptors in the left core of the nucleus accumbens Eur J Pharmacol 577:64–70 doi: 10.1016/j.ejphar.2007.08.028 [DOI] [PubMed] [Google Scholar]
- Jalewa J, Sharma MK, Gengler S, Holscher C (2017) A novel GLP-1/GIP dual receptor agonist protects from 6-OHDA lesion in a rat model of Parkinson’s disease Neuropharmacology 117:238–248 doi: 10.1016/j.neuropharm.2017.02.013 [DOI] [PubMed] [Google Scholar]
- Jang EY et al. (2017) The role of reactive oxygen species in methamphetamine self-administration and dopamine release in the nucleus accumbens Addict Biol doi: 10.1111/adb.12419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz JL, Su TP, Hiranita T, Hayashi T, Tanda G, Kopajtic T, Tsai SY (2011) A Role for Sigma Receptors in Stimulant Self Administration and Addiction Pharmaceuticals (Basel) 4:880–914 doi: 10.3390/ph4060880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly PH, Seviour PW, Iversen SD (1975) Amphetamine and apomorphine responses in the rat following 6-OHDA lesions of the nucleus accumbens septi and corpus striatum Brain Res 94:507–522 doi: 10.1016/0006-8993(75)90233-4 [DOI] [PubMed] [Google Scholar]
- Kramer PF, Williams JT (2016) Calcium Release from Stores Inhibits GIRK Cell Rep 17:3246–3255 doi: 10.1016/j.celrep.2016.11.076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto RR, Nguyen L, Kaushal N, Robson MJ (2014) Sigma (sigma) receptors as potential therapeutic targets to mitigate psychostimulant effects Adv Pharmacol 69:323–386 doi: 10.1016/B978-0-12-420118-7.00009-3 [DOI] [PubMed] [Google Scholar]
- Maurice T, Su TP (2009) The pharmacology of sigma-1 receptors Pharmacol Ther 124:195–206 doi: 10.1016/j.pharmthera.2009.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton TF, De La Garza R 2nd, Kalechstein AD, Nestor L (2005) Cocaine and methamphetamine produce different patterns of subjective and cardiovascular effects Pharmacol Biochem Behav 82:90–97 doi: 10.1016/j.pbb.2005.07.012 [DOI] [PubMed] [Google Scholar]
- Nguyen L, Kaushal N, Robson MJ, Matsumoto RR (2014) Sigma receptors as potential therapeutic targets for neuroprotection Eur J Pharmacol 743:42–47 doi: 10.1016/j.ejphar.2014.09.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruscher K, Wieloch T (2015) The involvement of the sigma-1 receptor in neurodegeneration and neurorestoration J Pharmacol Sci 127:30–35 doi: 10.1016/j.jphs.2014.11.011 [DOI] [PubMed] [Google Scholar]
- Saigusa T, Koshikawa N, Kitamura M, Kobayashi M (1993) Reevaluation of the two-component hypothesis for turning behaviour by manipulating activities in the striatum and the nucleus accumbens of intact rats Eur J Pharmacol 237:161–168 [DOI] [PubMed] [Google Scholar]
- Sambo DO et al. (2017) The sigma-1 receptor modulates methamphetamine dysregulation of dopamine neurotransmission Nat Commun 8:2228 doi: 10.1038/s41467-017-02087-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seecof R, Tennant FS Jr. (1986) Subjective perceptions to the intravenous “rush” of heroin and cocaine in opioid addicts Am J Drug Alcohol Abuse 12:79–87 doi: 10.3109/00952998609083744 [DOI] [PubMed] [Google Scholar]
- Seidler NW, Jona I, Vegh M, Martonosi A (1989) Cyclopiazonic acid is a specific inhibitor of the Ca2+-ATPase of sarcoplasmic reticulum J Biol Chem 264:17816–17823 [PubMed] [Google Scholar]
- Siciliano CA, Calipari ES, Ferris MJ, Jones SR (2014) Biphasic mechanisms of amphetamine action at the dopamine terminal J Neurosci 34:5575–5582 doi: 10.1523/JNEUROSCI.4050-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solis E Jr. (2017) Electrophysiological Actions of Synthetic Cathinones on Monoamine Transporters Curr Top Behav Neurosci 32:73–92 doi: 10.1007/7854_2016_39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffensen SC, Taylor SR, Horton ML, Barber EN, Lyle LT, Stobbs SH, Allison DW (2008) Cocaine disinhibits dopamine neurons in the ventral tegmental area via use-dependent blockade of GABA neuron voltage-sensitive sodium channels Eur J Neurosci 28:2028–2040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su TP, Hayashi T, Maurice T, Buch S, Ruoho AE (2010) The sigma-1 receptor chaperone as an inter-organelle signaling modulator Trends Pharmacol Sci 31:557–566 doi: 10.1016/j.tips.2010.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudhof TC (2004) The synaptic vesicle cycle Annu Rev Neurosci 27:509–547 doi: 10.1146/annurev.neuro.26.041002.131412 [DOI] [PubMed] [Google Scholar]
- Sugimoto K, Okamura K, Tanaka H, Takashima S, Ochi H, Yamamoto T, Matoba R (2009) Methamphetamine directly accelerates beating rate in cardiomyocytes by increasing Ca(2+) entry via L-type Ca(2+) channel Biochem Biophys Res Commun 390:1214–1220 doi: 10.1016/j.bbrc.2009.10.124 [DOI] [PubMed] [Google Scholar]
- Tsien RY (1981) A non-disruptive technique for loading calcium buffers and indicators into cells Nature 290:527–528 doi: 10.1038/290527a0 [DOI] [PubMed] [Google Scholar]
- Uramura K, Yada T, Muroya S, Shioda S, Shiratani T, Takigawa M (2000) Methamphetamine induces cytosolic Ca2+ oscillations in the VTA dopamine neurons Neuroreport 11:1057–1061 [DOI] [PubMed] [Google Scholar]
- Wasik A, Romanska I, Zelek-Molik A, Antkiewicz-Michaluk L (2018) Multiple Administration of Endogenous Amines TIQ and 1MeTIQ Protects Against a 6-OHDA-Induced Essential Fall of Dopamine Release in the Rat Striatum: In Vivo Microdialysis Study Neurotox Res 33:523–531 doi: 10.1007/s12640-017-9824-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wightman RM, Zimmerman JB (1990) Control of dopamine extracellular concentration in rat striatum by impulse flow and uptake Brain Res Brain Res Rev 15:135–144 doi: 10.1016/0165-0173(90)90015-g [DOI] [PubMed] [Google Scholar]
- Yorgason JT, Espana RA, Jones SR (2011a) Demon voltammetry and analysis software: analysis of cocaine-induced alterations in dopamine signaling using multiple kinetic measures J Neurosci Methods 202:158–164 doi:S0165–0270(11)00128–2 [pii] 10.1016/j.jneumeth.2011.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yorgason JT, Jones SR, Espana RA (2011b) Low and high affinity dopamine transporter inhibitors block dopamine uptake within 5 sec of intravenous injection Neuroscience 182:125–132 doi: 10.1016/j.neuroscience.2011.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yorgason JT, Zeppenfeld DM, Williams JT (2017) Cholinergic Interneurons Underlie Spontaneous Dopamine Release in Nucleus Accumbens J Neurosci 37:2086–2096 doi: 10.1523/JNEUROSCI.3064-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young A, Assey KS, Sturkie CD, West FD, Machacek DW, Stice SL (2010) Glial cell line-derived neurotrophic factor enhances in vitro differentiation of mid-/hindbrain neural progenitor cells to dopaminergic-like neurons J Neurosci Res 88:3222–3232 doi: 10.1002/jnr.22499 [DOI] [PubMed] [Google Scholar]
- Yu SJ, Wu KJ, Bae EK, Hsu MJ, Richie CT, Harvey BK, Wang Y (2016) Methamphetamine induces a rapid increase of intracellular Ca(++) levels in neurons overexpressing GCaMP5 Addict Biol 21:255–266 doi: 10.1111/adb.12193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zernig G, Giacomuzzi S, Riemer Y, Wakonigg G, Sturm K, Saria A (2003) Intravenous drug injection habits: drug users’ self-reports versus researchers’ perception Pharmacology 68:49–56 doi: 10.1159/000068731 [DOI] [PubMed] [Google Scholar]
- Zhu G, Okada M, Yoshida S, Hirose S, Kaneko S (2004) Both 3,4-dihydroxyphenylalanine and dopamine releases are regulated by Ca2+-induced Ca2+ releasing system in rat striatum Neurosci Lett 362:244–248 doi: 10.1016/j.neulet.2004.03.031 [DOI] [PubMed] [Google Scholar]