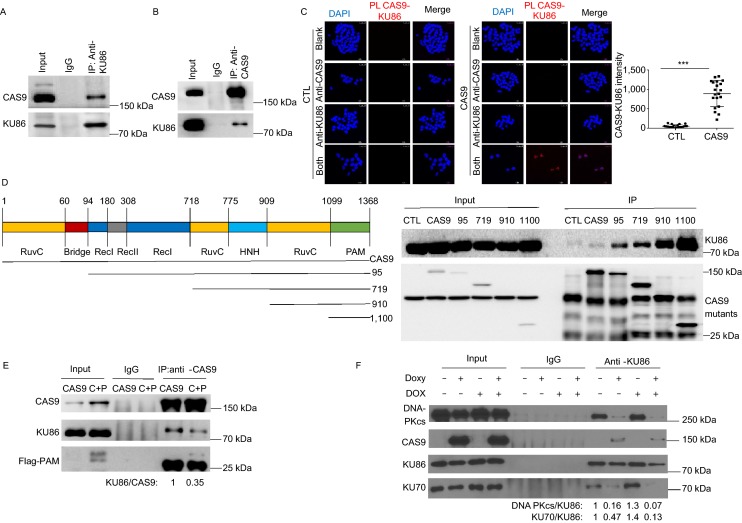
Figure 4.
CAS9 interacts with KU86 and disrupts the formation of DNA-PK. (A and B) Reciprocal immunoprecipitation shows the interaction between CAS9 and KU86. Protein extracts from hESCs expressing CAS9 were immunoprecipitated with anti-KU86 (A) or anti-CAS9 (B), and immune precipitates were analyzed for the presence of KU86 and CAS9. (C) The interaction between CAS9 and KU86 was confirmed by the proximity ligation analysis (PLA). Cell nucleus were revealed by DAPI (Blue) staining and the CAS9-KU86 interaction indicated by red color. Scale bar, 25 µm. Unpaired t test. n = 20. Data are presented as mean value ± SD. ***P < 0.001. (D) Mapping the domain of CAS9 involved in the interaction with KU86. The Flag-tagged deletional mutants of CAS9 expressed in 293FT cells were immunoprecipitated with anti-flag antibody. Immune precipitates were analyzed for the presence of CAS9 mutants and KU86. (E) The expression of the PAM domain (1100) of CAS9 disrupted the interaction between CAS9 and KU86. The levels of CAS9, KU86, PAM in the input and immunoprecipitate were analyzed by Western blot. The ratio of CAS9 versus KU86 in the immunoprecipitate is shown at the bottom. (F) CAS9 disrupts the formation of DNA-PK complex. Protein extracts of cells in the presence and absence of CAS9 and DOX treatment were immunoprecipitated with anti-KU86 antibody. The levels of KU70, DNA-PKcs and CAS9 in the immunoprecipitate were analyzed. The relative ratios of DNA-PKcs versus KU86 or KU70 versus KU86 are indicated