Figure 1.
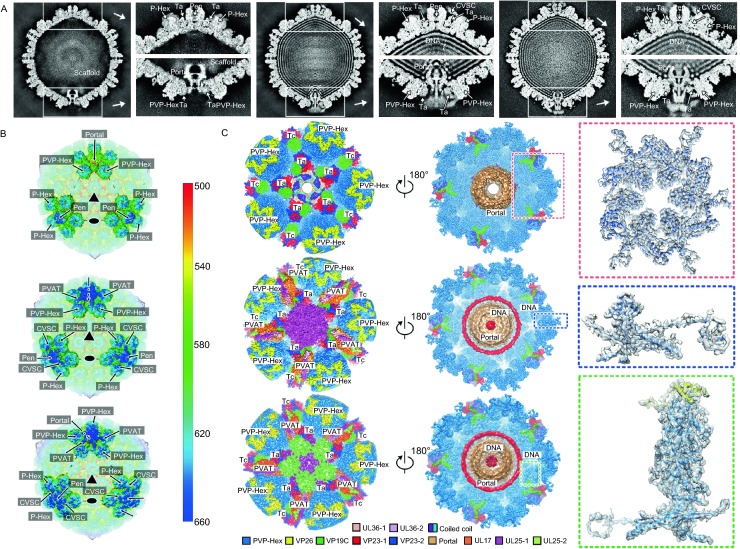
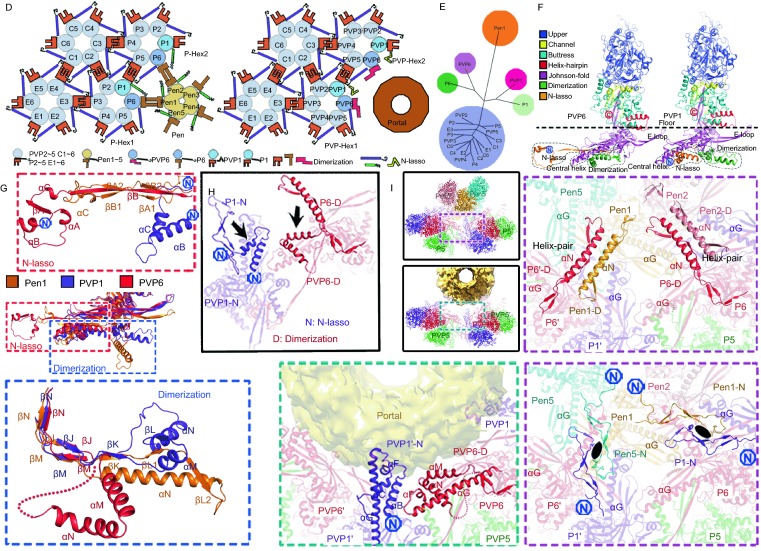
Overall structures of the portal vertex and organization of VP5. (A) Central slices of asymmetric reconstructions of HSV-2 B-, C- and virion-capsids. The inserts show the densities of the penton and portal vertices. P-Hex, PVP-Hex, Ta, and Pen denote peripentonal hexons, portal vertex periportal hexons, the triplex A and pentons, respectively. (B) Locations of portal and penton vertex components on the radially colored asymmetric reconstructions of B-, C- and virion-capsids. The pseudo three-fold and two-fold icosahedral symmetry axes are marked as triangles and ellipses, respectively. (C) Cryo-EM maps of B-, C-capsid and viron portal vertices. The insets show the density maps and related atomic models, which illustrate polypeptide backbone and many bulky side chains features. (D) Schematic diagram of the binding mode of different capsomers. Domains with significant conformational differences (Dimerization and N-lasso domains) are highlighted. (E) Structure-based phylogenetic tree of 22 types of VP5 present in the capsid. (F) Ribbon diagram of PVP1 and PVP6. N and C termini are labeled, and major conformational changes are marked with dashed lines. (G) A superposition of the floor of three representative VP5s (Pen1, PVP1, and PVP6). Three types of the N-lasso and dimerization domains are enlarged in red and blue insets, respectively. (H) A superposition of P1 and P6 structures onto PVP1 and PVP6 structures, highlighting the conformational retractions of the PVP1 N-lasso and PVP6 dimerization domains. (I) Comparisons of the intercapsomer interactions between penton and P-Hexs and between portal and PVP-Hexs. The insets show the conformational changes at the vertices. Five helix-pairs and a set of five quasi-equivalent two-fold interactions contributing to the majority of the interactions of P-Hex and penton are replaced by five sets of two four-helix bundles in the portal vertex. The quasi-equivalent two folds axes are marked with ellipses. D and N denote Dimerization and N-lasso domains, respectively