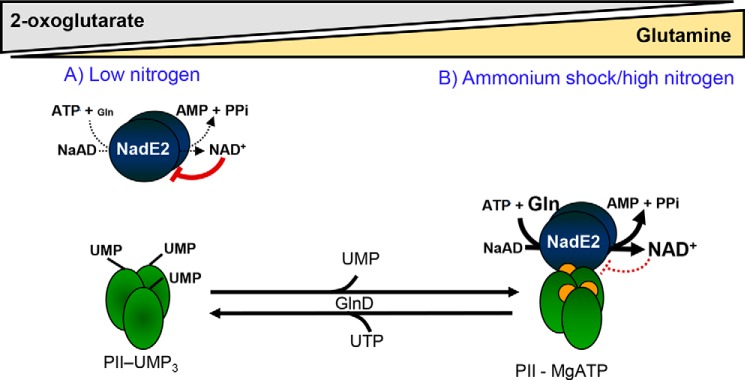
Figure 7.
The PII proteins act as a dissociable regulatory subunit of NadE2Gln to pace NAD+ production accordingly to the availability of l-glutamine and 2-OG. A, when nitrogen is limited, cell growth is arrested, the intracellular levels of 2-OG are high, and glutamine are low. PII is fully uridylylated and does not interact with NadE2, resulting in strong feedback negative inhibition of NadE2 by NAD+. B, when ammonium becomes available, glutamine rises and 2-OG drops, PII is deuridylylated (reaction catalyzed by the GlnD enzyme in response to high glutamine) and bound to MgATP and/or ADP (orange dots). Under this condition, PII interacts with NadE2 relieving NAD+ inhibition and thus allowing higher NAD+ production rates to feed the demands of active cell growth.