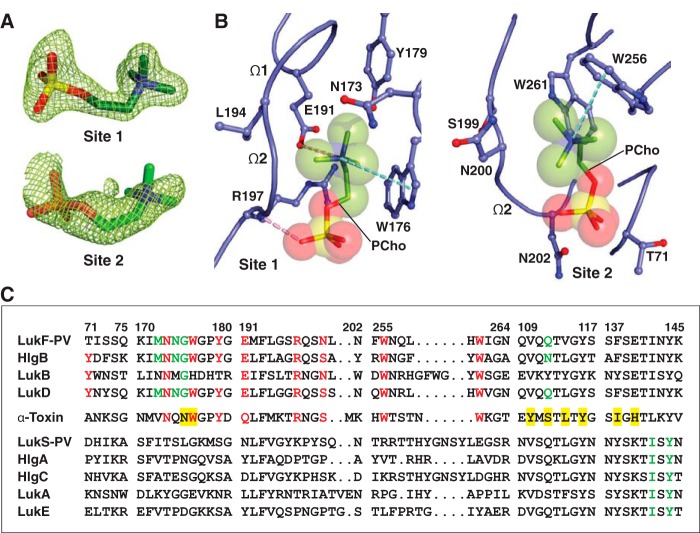
Figure 2.
Structure of C14PC-bound Luk-PV. A, 2Fo − Fc electron density map shown as green mesh around the two PCho moieties contoured at 1.5σ (site 1) and 1.0σ (site 2). B, molecular interactions in the C14PC-LukF-PV complex, with the PCho moieties shown in stick format with transparent CPK spheres. The side chains of residues that make direct contacts with the PCho moieties are shown in ball-and-stick format. The two binding sites and the Ω1 and Ω2 loops are labeled. Cation-π interactions are represented as green dotted lines. Hydrogen bonds and salt bridges are shown as pink dotted lines. C, sequence alignment for members of the α-hemolysin toxin subfamily around the regions of the three PCho-binding sites (see “Results” for details). Four segments of the rim domain (residues 71–75, 170–180, 191–202, and 255–264) and two segments of the stem domain (residues 109–117 and 137–145) are delineated by spaces and numbered according to the mature LukF-PV protein. Conserved residues at the two binding sites on the rim domain are highlighted in red. Conserved residues that constitute the interprotomer binding sites on the PVL heterooctamer and the α-toxin heptamer are highlighted with a green and yellow background, respectively.