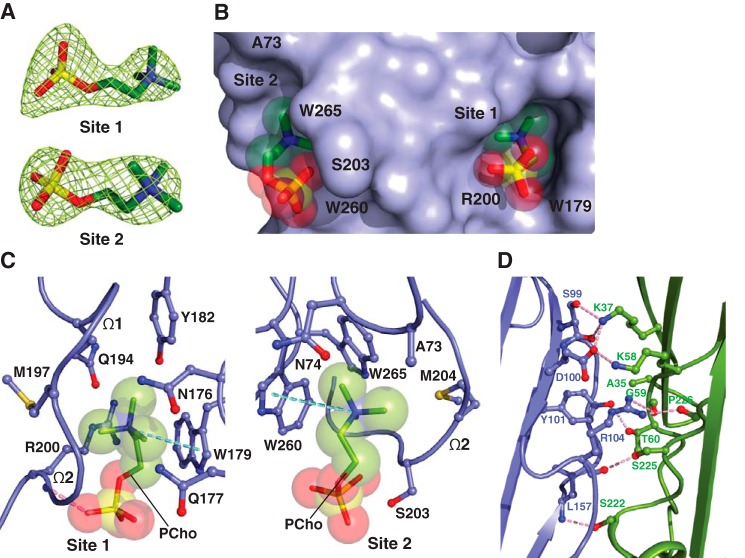
Figure 3.
The two adjacent PC-binding pockets on monomeric α-toxinH35A. A, 2Fo − Fc electron density map (green mesh) for the two PCho moieties contoured at 1.2σ (site 1) and 1.0σ (site 2). B, surface representation of the C14PC-α-toxinH35A complex viewed parallel to the membrane, with the PCho moieties shown in stick format with transparent CPK spheres. The two binding sites are labeled. The locations of key binding site residues are indicated. C, close-up view of the two adjacent PCho-binding pockets, with the PCho moieties displayed in stick format with transparent CPK spheres. Residues that make direct side chain contacts with the PCho moieties are shown in ball-and-stick format. The Ω1 and Ω2 loops are labeled. Cation-π interactions are represented as green dotted lines. Hydrogen bonds are shown as pink dotted lines. D, close-up view of the interface between the two independent α-toxinH35A monomers (blue and green, respectively) in the asymmetric unit. Hydrogen bonds and salt bridges near the His35→Ala mutation site are depicted as pink dotted lines.