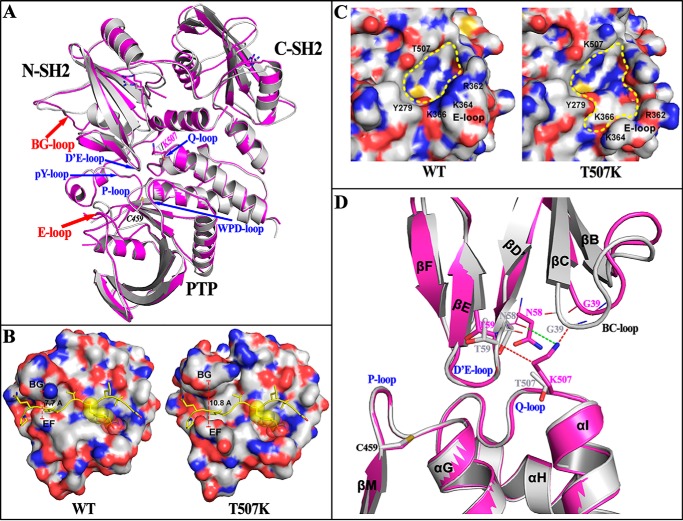
Figure 1.
Structural perturbations in SHP2 introduced by the T507K mutation. A, SHP2/T507K (purple) shows similar overall structure compared with WT SHP2 (gray, PDB code 4DGP) in the closed conformation, but with structural alterations around the BG-loop and E-loop, and its N-terminal SH2 domains shifting slightly away from the PTP domain. B, conformational change of the BG-loop leads to a more open BG/EF gate in SHP2/T507K, which potentially benefits the binding of N-SH2 domain with adapter proteins for the T507K mutant. To illustrate this effect, the structure of N-SH2·phosphopeptide (PDB code 1AYA) was superimposed, respectively, onto the N-SH2 domain in WT or T507K mutant SHP2, and the phosphopeptide (yellow ribbon with side chain) was shown to represent a bound conformation. C, conformational change for the E-loop in SHP2/T507K alters the catalytic active site pocket. D, potential driving force for SH2 domain shifting in SHP2/T507K. The T507K structure (purple) was superimposed onto the WT SHP2 structure (gray). The steric repulsions from the T507K mutation are presented by a red dashed line, and the newly-added hydrogen bond is indicated by a green dashed line.