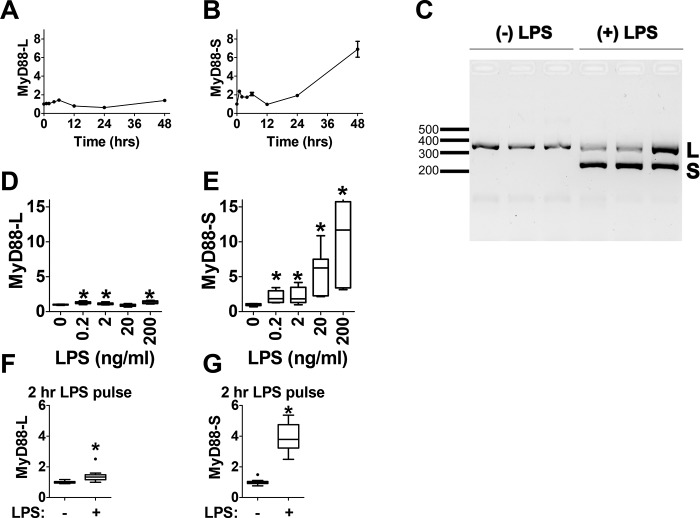
Figure 1.
LPS induces MyD88-S expression in mouse macrophages. A and B, analysis of MyD88-L and MyD88-S mRNA expression at multiple time points during LPS exposure in RAW264.7 macrophages. Macrophages were treated with 200 ng/ml LPS or were left untreated, and MyD88 isoform levels were monitored at each time point relative to the untreated control using isoform-specific qPCR. C, RAW 264.7 cells were exposed to LPS (200 ng/ml) for 48 h or not exposed to LPS as a control. Total RNAs were then harvested, RT-PCR was performed using primers that bracket MyD88 exon 2, and the resulting PCR products were subjected to agarose gel electrophoresis. This allowed the simultaneous identification of PCR products corresponding to MyD88-L (369 bp) and MyD88-S (234 bp). L, MyD88-L; S, MyD88-S. Each lane represents an independent biological replicate. D and E, analysis of MyD88-L (D) and MyD88-S (E) mRNA expression at multiple LPS doses; RAW264.7 macrophages were treated with the indicated concentrations of LPS for 48 h, and MyD88 isoform expression was monitored by qPCR. F and G, MyD88-L and MyD88-S mRNA expression in LPS pulse–treated macrophages. RAW264.7 macrophages were treated with 200 ng/ml LPS for 2 h, the medium was then changed to medium lacking LPS, and the cells were incubated for an additional 22 h (24 h total) prior to harvesting RNA for isoform-specific qPCR. All qPCR data represent a minimum of three biological replicates. mRNA levels for each isoform are normalized to 1 in the absence of LPS. *, p < 0.05.