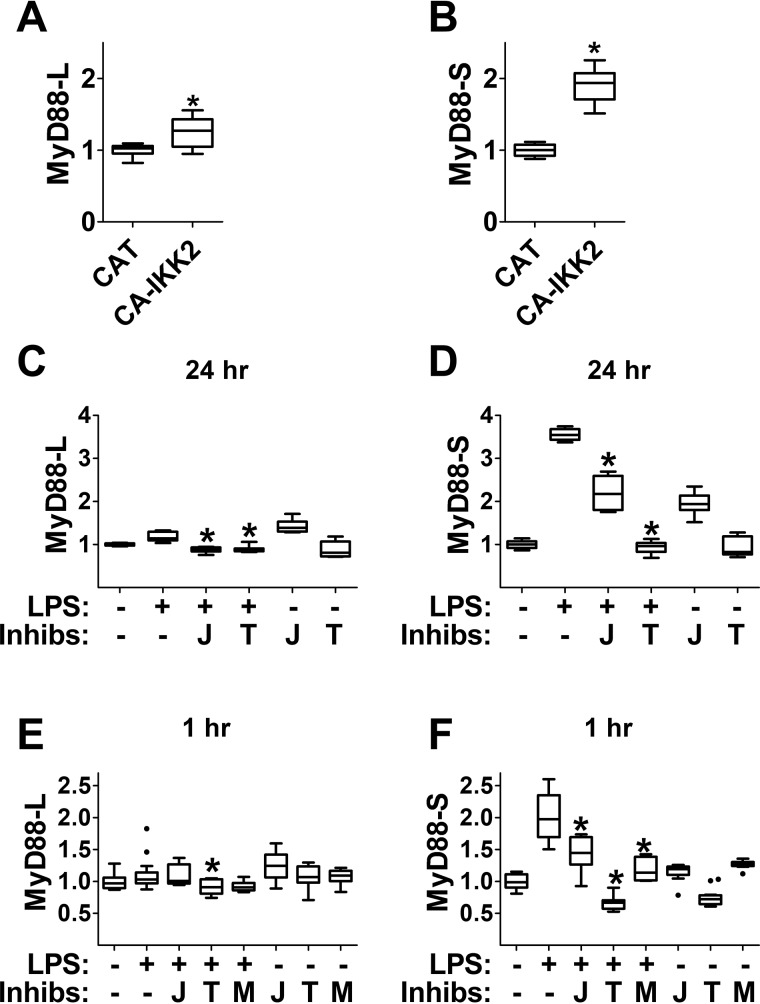
Figure 6.
NF-κB regulates MyD88 alternative pre-mRNA splicing. A and B, RAW264.7 macrophages were transiently transfected either with negative control protein CAT or with a plasmid overexpressing constitutively activated (CA) IKK2 (IKK2-S177E-S181E). 48 h after transfection, MyD88-L and MyD88-S mRNA levels were monitored by qPCR. C and D, RAW264.7 cells were pulse-treated with 200 ng/ml LPS for 2 h (or were left untreated as a control) in either the presence or absence of inhibitors of NF-κB (J = JSH23, T = TPCA1, M = MG132). After a further 22-h incubation with or without these inhibitors, RNA was collected, and MyD88-L and MyD88-S mRNA levels were assessed by qPCR. To prevent cell death, the NF-κB inhibition studies were performed in the presence of two apoptosis inhibitors, Z-VAD-fmk and necrostatin. E and F, RAW264.7 cells were treated with 200 ng/ml LPS for 1 h (or were left untreated as a control) in either the presence or absence of the indicated inhibitors of NF-κB. RNA was then collected, and MyD88-L and MyD88-S mRNA levels were assessed by qPCR. All experiments represent a minimum of three independent biological replicates. Data are normalized so that MyD88-L or MyD88-S expression in the absence of LPS is set to 1. *, p < 0.05.