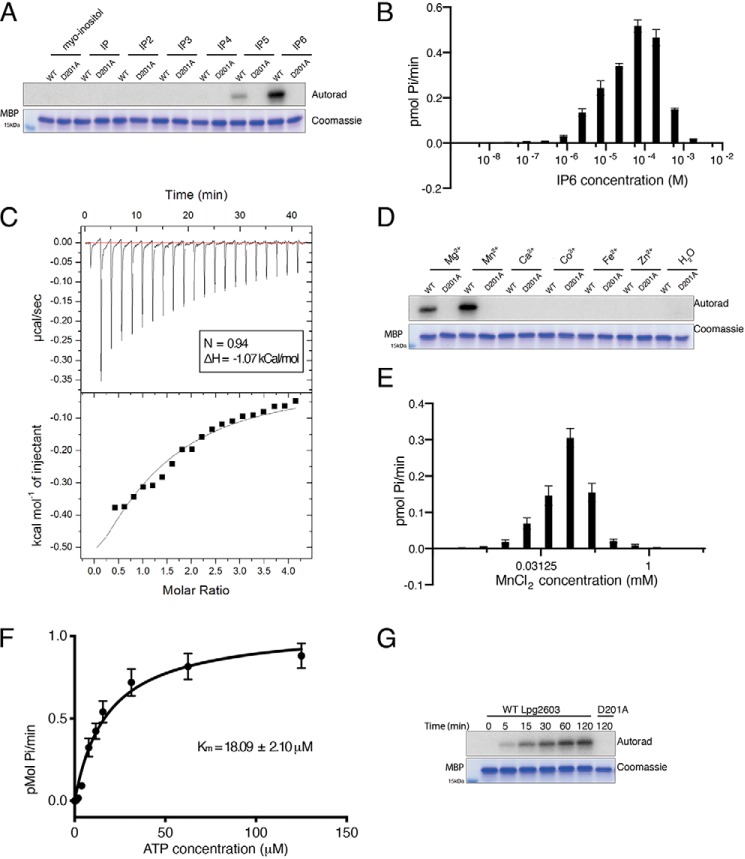
Figure 2.
Lpg2603 activity requires IP6 and Mn2+. A, in vitro kinase assay showing incorporation of 32P from [γ-32P]ATP into MBP by Lpg2603 in the presence of myo-inositol, inositol phosphate (IP), inositol diphosphate (IP2), inositol triphosphate (IP3), inositol tetraphosphate (IP4), inositol pentaphosphate (IP5), and IP6. The reactions were terminated and analyzed as in Fig. 1B. The results are representative of three independent experiments. B, incorporation of γ-32P from [γ-32P]ATP into MBP by Lpg2603 with increasing concentrations of IP6. The reactions were terminated after 10 min by the addition of EDTA, the products were resolved by SDS-PAGE, and Coomassie-stained MBP bands were excised for scintillation counting. The results are representative of three independent experiments. The error bars represent standard deviation from three replicates from an individual experiment. C, representative isothermal calorimetry data for Lpg2603 binding to IP6. Lpg2603 is at 200 μm in the cell, and IP6 is at 8 mm in the titration syringe for a final molar ratio of 1:4. Best fit parameters were as follows: n = 0.94; ΔH = −1.07 kCal/mol. D, in vitro kinase assay showing incorporation of γ-32P from [γ-32P]ATP into MBP by Lpg2603 in the presence or absence of MgCl2, MnCl2, CaCl2, CoCl2, FeCl2, and ZnCl2. The reactions were terminated and analyzed as in Fig. 1B. The results are representative of three independent experiments. E, incorporation of γ-32P from [γ-32P]ATP into MBP by Lpg2603 with varying concentrations of MnCl2. The reactions were terminated and analyzed as in B. The results are representative of three independent experiments. The error bars represent standard deviation from three replicates from an individual experiment. F, kinetic analysis depicting the concentration dependence of Mn2+/ATP on the rate of MBP phosphorylation by Lpg2603. Km for Mn2+/ATP = 18.09 ± 2.10 μm; Vmax = 1.05 ± 0.04 pmol/min Reactions were terminated and analyzed as in B. The results are representative of three independent experiments. The error bars represent standard error of the mean from three replicates from an individual experiment. Kinetic measurements were fitted into the Michaelis–Menten equation using GraphPad Prism. G, time-dependent incorporation of γ-32P from [γ-32P]ATP into MBP by Lpg2603. The reactions were terminated and analyzed as in Fig. 1B. The results are representative of three independent experiments.