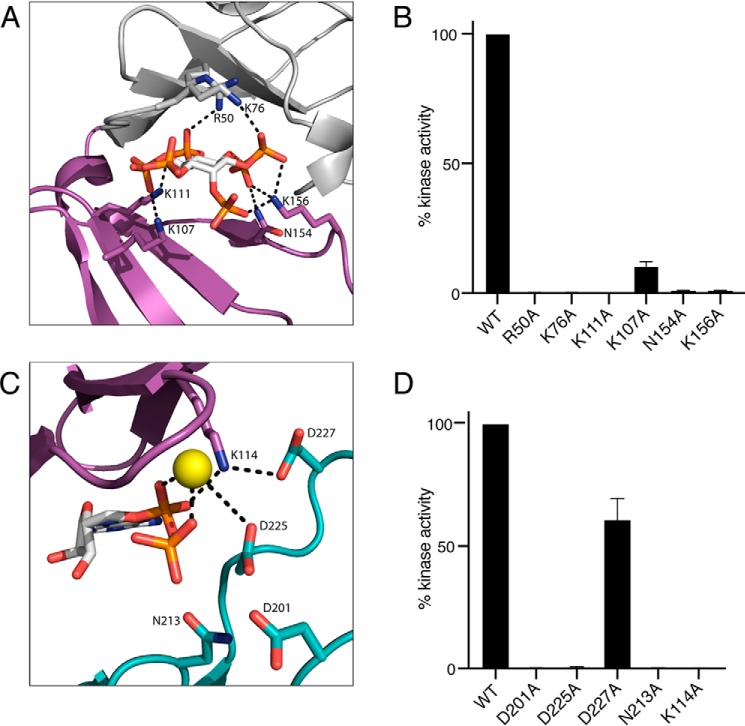
Figure 4.
Mutational analysis highlights residues in Lpg2603 important for catalysis. A, enlarged image of IP6-binding pocket showing residues and interactions important for IP6 binding. IP6 is shown in ball-and-stick form, colored according to atom. B, activity of Lpg2603 or mutants were assayed as in Fig. 2B. Activity is expressed relative to the WT enzyme. The results are representative of three independent experiments. The error bars represent standard deviation. C, enlarged image of the nucleotide-binding pocket showing residues and interactions important for ADP binding and catalysis. The bound ADP is shown in ball-and-stick form, colored according to atom. Mn2+ ion is shown as a yellow sphere. Note that Lys114 lies within the VAIK motif common in protein kinases. D, Lpg2603 or mutants were assayed as in Fig. 2B. Activity is expressed relative to the WT enzyme. The results are representative of three independent experiments. The error bars represent standard deviation.