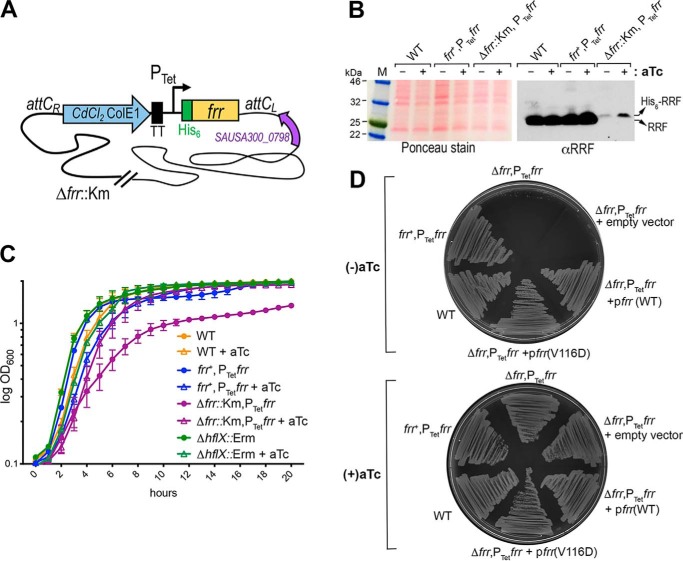
Figure 3.
In vivo phenotypes of the RRF-depleted strain. A, construction of the RRF-depleted strain. His6-tagged frr was first placed under the control of a tetracycline (Tet)-inducible promoter on a cadmium chloride (CdCl2)-resistant integration plasmid. The plasmid was integrated into the chromosomal attC site, resulting in an S. aureus strain carrying an inducible frr (PTetfrr) and a native copy of frr (frr+). The WT copy of frr was deleted (Δfrr::Km, confers kanamycin resistance) from the frr+,PTetfrr strain by homologous recombination in the presence of 400 ng/ml of aTc. TT, transcriptional terminator. B, relative expression levels of native RRF and aTc-inducible His6-RRF. The expression of RRF was analyzed by immunoblotting with anti-RRF (1:4,000 dilutions). RRF was expressed from the native locus at a much higher level than the His6-RRF. His6-RRF migrated slightly more slowly than the native RRF. C, growth defects of RRF depletion in liquid media. S. aureus cells were grown in TSB at 37 °C with and without aTc. Real-time cell density was monitored on a TECAN plate reader according to the absorbance at 600 nm. The addition of aTc slightly suppressed bacterial growth in all tested strains, but it restored the growth of the RRF-depleted strain to levels comparable with those in other strains with aTc supplementation. Error bars are S.E. from three independent experiments, and each experiment included five replicates. D, the growth defects of RRF depletion were more significant on solid agar plates. Single colonies of different S. aureus strains were streaked on BactoTM agar plates containing TSB base, and bacterial growth was recorded after 16 h of incubation at 37 °C. The RRF-depleted strain (Δfrr,PTetfrr) and its derivative carrying the empty pLI50 plasmid only formed a few microcolonies in the absence of aTc. WT frr and frr(V116D) expressed in the pLI50 plasmid fully rescued the growth defects of Δfrr,PTetfrr.