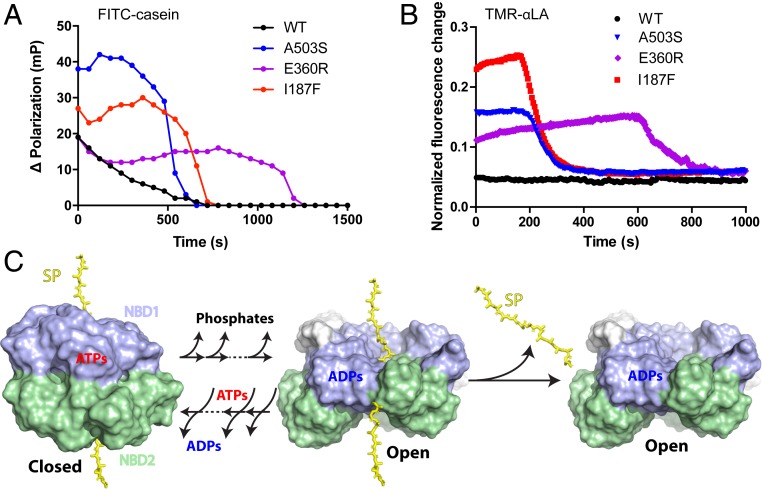
Fig. 7.
The potentiating mutations promote substrate retention during active turnover. (A) Increased ΔPolarization signal due to retention of FITC-casein substrate during active ATP turnover. (B) Increased fluorescence due to retention of TMR-labeled αLA substrate during active ATP turnover. Low signal for WT Hsp104 (black) reflects the low steady-state level of bound-substrate due to substrate loss from the open state during ATP cycling. Increased steady state signal for the potentiated mutants reflects improved substrate retention due to less cycle time in the open state. Control experiments with Tyr pore loop mutants indicate that these signals reflect active substrate protein processing through the axial channel (SI Appendix, Fig. S8D) although the polarization signal probably also includes external protein. Final signal decay is due to ATP exhaustion, ADP accumulation, and substrate loss. Time in the steady-state substrate-bound condition before decay matches the expected exhaustion of the ATP regenerating capacity, dependent on the known ATP utilization rate (Table 1). The fluorescence traces (three replicates) are normalized to the high value obtained in the presence of ATPγS (SI Appendix, Fig. S8D). Pertinent background signals are subtracted. (4 μM Hsp104 in protomer; 10 mM phophoenolpyruvate for experiments shown in A and 5 mM for B; initial free ATP 10 mM; see also SI Appendix, Materials and Methods). (C) The kinetic competition during ATP/ADP cycling between loss of substrate protein from the ADP open state and ATP rebinding to induce the closed state. The transition between the closed and open states may involve multiple ATP hydrolysis or binding steps. The model omits possible multiple intermediate states bound with mixed ADP/ATP for simplicity. ADP release from the open state determines the fraction of time Hsp104 spend in this state. Slower ADP release leads to more time in the open state and, in turn, more substrate protein (SP) lost.